| |
Lecanemab in Early Alzheimer's Disease
|
| |
| |
Download the PDF here
Current therapeutic agents for Alzheimer's disease-related dementia temporarily improve symptoms but do not alter the underlying disease course.1,2 Some evidence suggests that amyloid removal slows the progression of disease.3 One anti-amyloid antibody (aducanumab) has received accelerated approval from the Food and Drug Administration.
Lecanemab is a humanized monoclonal antibody that binds with high affinity to soluble amyloid-beta (Aβ) protofibrils, which have been shown to be more toxic to neurons than monomers or insoluble fibrils.4-14 A phase 2b, dose-finding trial involving 854 participants with early Alzheimer's disease did not show a significant difference between lecanemab and placebo in a Bayesian analysis of 12-month change in a composite score (primary end point). However, analyses at 18 months showed dose- and time-dependent clearance of amyloid with lecanemab, and the drug was associated with less clinical decline on some measures than placebo. In that trial, intravenous administration of 10 mg of lecanemab per kilogram of body weight every 2 weeks was identified as an appropriate dose, with a 9.9% incidence (<3% symptomatic) of amyloid-related imaging abnormalities (ARIA) with edema or effusions (ARIA-E).15 We conducted a phase 3 trial (Clarity AD) to determine the safety and efficacy of lecanemab in participants with early Alzheimer's disease.
Report shares new details about death possibly linked to experimental Alzheimer's drug
https://www.vox.com/policy-and-politics/2023/1/6/23540617/alzheimers-lecanemab-vs-aducanemab-fda-approval
But lecanemab's quest for approval is shadowed by the previous approval of another Alzheimer's drug to get accelerated approval: aducanemab, sold as Aduhelm.
Abstract
Background
The accumulation of soluble and insoluble aggregated amyloid-beta (Aβ) may initiate or potentiate pathologic processes in Alzheimer's disease. Lecanemab, a humanized IgG1 monoclonal antibody that binds with high affinity to Aβ soluble protofibrils, is being tested in persons with early Alzheimer's disease.
Methods
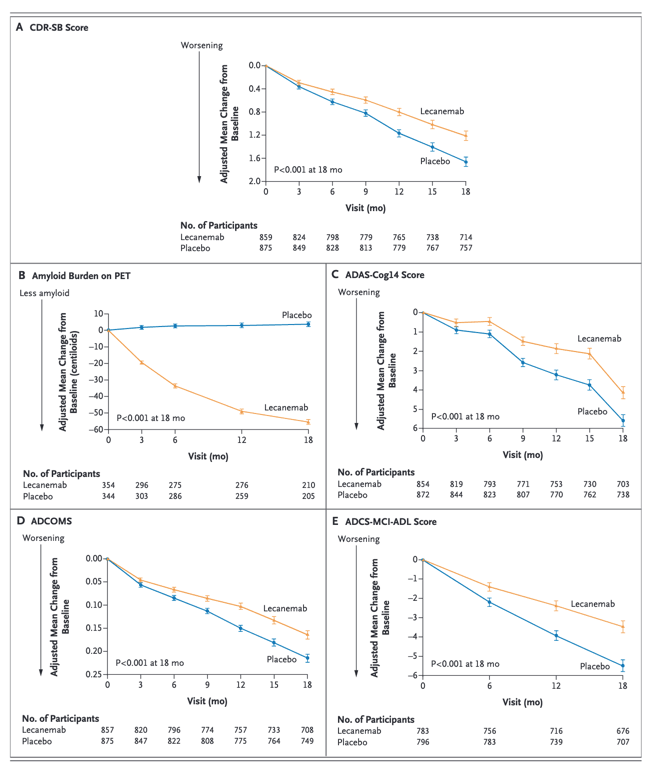
We conducted an 18-month, multicenter, double-blind, phase 3 trial involving persons 50 to 90 years of age with early Alzheimer's disease (mild cognitive impairment or mild dementia due to Alzheimer's disease) with evidence of amyloid on positron-emission tomography (PET) or by cerebrospinal fluid testing. Participants were randomly assigned in a 1:1 ratio to receive intravenous lecanemab (10 mg per kilogram of body weight every 2 weeks) or placebo. The primary end point was the change from baseline at 18 months in the score on the Clinical Dementia Rating-Sum of Boxes (CDR-SB; range, 0 to 18, with higher scores indicating greater impairment). Key secondary end points were the change in amyloid burden on PET, the score on the 14-item cognitive subscale of the Alzheimer's Disease Assessment Scale (ADAS-cog14; range, 0 to 90; higher scores indicate greater impairment), the Alzheimer's Disease Composite Score (ADCOMS; range, 0 to 1.97; higher scores indicate greater impairment), and the score on the Alzheimer's Disease Cooperative Study-Activities of Daily Living Scale for Mild Cognitive Impairment (ADCS-MCI-ADL; range, 0 to 53; lower scores indicate greater impairment).
Results
A total of 1795 participants were enrolled, with 898 assigned to receive lecanemab and 897 to receive placebo. The mean CDR-SB score at baseline was approximately 3.2 in both groups. The adjusted least-squares mean change from baseline at 18 months was 1.21 with lecanemab and 1.66 with placebo (difference, -0.45; 95% confidence interval [CI], -0.67 to -0.23; P<0.001). In a substudy involving 698 participants, there were greater reductions in brain amyloid burden with lecanemab than with placebo (difference, -59.1 centiloids; 95% CI, -62.6 to -55.6). Other mean differences between the two groups in the change from baseline favoring lecanemab were as follows: for the ADAS-cog14 score, -1.44 (95% CI, -2.27 to -0.61; P<0.001); for the ADCOMS, -0.050 (95% CI, -0.074 to -0.027; P<0.001); and for the ADCS-MCI-ADL score, 2.0 (95% CI, 1.2 to 2.8; P<0.001). Lecanemab resulted in infusion-related reactions in 26.4% of the participants and amyloid-related imaging abnormalities with edema or effusions in 12.6%.
Conclusions
Lecanemab reduced markers of amyloid in early Alzheimer's disease and resulted in moderately less decline on measures of cognition and function than placebo at 18 months but was associated with adverse events. Longer trials are warranted to determine the efficacy and safety of lecanemab in early Alzheimer's disease. (Funded by Eisai and Biogen; Clarity AD ClinicalTrials.gov number, NCT03887455. opens in new tab.)
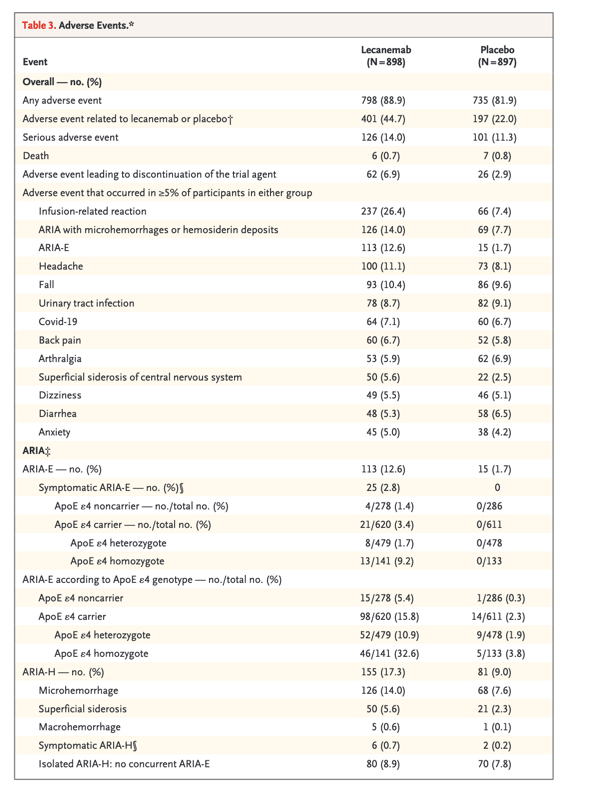
Safety
Deaths occurred in 0.7% of the participants in the lecanemab group and 0.8% of those in the placebo group ( Table 3). No deaths were considered by the investigators to be related to lecanemab or occurred with ARIA. Serious adverse events occurred in 14.0% of the participants in the lecanemab group and 11.3% of those in the placebo group. The most commonly reported serious adverse events were infusion-related reactions (in 1.2% of the participants in the lecanemab group and 0 participants in the placebo group), ARIA-E (in 0.8% and 0, respectively), atrial fibrillation (in 0.7% and 0.3%), syncope (in 0.7% and 0.1%), and angina pectoris (in 0.7% and 0). The overall incidence of adverse events was similar in the two groups (Table 3). Adverse events leading to discontinuation of the trial agent occurred in 6.9% of the participants in the lecanemab group and 2.9% of those in the placebo group. The most common adverse events (affecting >10% of the participants) in the lecanemab group were infusion-related reactions (26.4% with lecanemab and 7.4% with placebo); ARIA with cerebral microhemorrhages, cerebral macrohemorrhages, or superficial siderosis (ARIA-H; 17.3% with lecanemab and 9.0% with placebo); ARIA-E (12.6% with lecanemab and 1.7% with placebo); headache (11.1% with lecanemab and 8.1% with placebo); and falls (10.4% with lecanemab and 9.6% with placebo). Infusion-related reactions were largely mild to moderate (grade 1 or 2, 96%) and occurred with the first dose (75%). A total of 56% of the participants did not take preventative medications (i.e., nonsteroidal antiinflammatory drugs, antihistamines, or glucocorticoids) for infusion-related reactions. Of those who took preventative medications for subsequent doses, 63% did not have additional reactions.
Events of ARIA-E with lecanemab were mostly mild to moderate (91%) on the basis of central reading of imaging with the use of protocol definitions. These events were mostly asymptomatic (78%), occurred during the first 3 months of the treatment period (71%), and resolved within 4 months after detection (81%). A total of 2.8% of the participants in the lecanemab group had symptomatic ARIA-E; commonly reported symptoms were headache, visual disturbance, and confusion. The incidence of isolated ARIA-H (i.e., ARIA-H in participants who did not also have ARIA-E) was 8.9% in the lecanemab group and 7.8% in the placebo group. The incidence of isolated symptomatic ARIA-H was 0.7% in the lecanemab group and 0.2% in the placebo group. The most common symptom associated with isolated symptomatic ARIA-H was dizziness. Macrohemorrhage occurred in 5 of 898 participants (0.6%) in the lecanemab group and 1 of 897 participants (0.1%) in the placebo group. ARIA-H that occurred with ARIA-E tended to occur early (within 6 months). Isolated ARIA-H occurred throughout the trial. ARIA-E and ARIA-H were numerically less common among ApoE ε4 noncarriers than among carriers, with higher frequency among ApoE ε4 homozygotes than among ApoE ε4 heterozygotes (Table 3).
|
|
| |
| |
|
|
|