| |
Lenacapavir administered every 26 weeks or daily in combination with oral daily antiretroviral therapy for initial treatment of HIV: a randomised, open-label, active-controlled, phase 2 trial
|
| |
| |
Download the PDF here
Arc of Aging with HIV 1996-2022: from promise to disappointment & despair - Download the PDF here
January, 2023
IAS Long-acting subcutaneous lenacapavir dosed every 6 months as part of a combination regimen in treatment-naïve people with HIV: interim 16-week results of a randomized, open-label, phase 2 induction-maintenance study (CALIBRATE)
Sunlenca® (lenacapavir) Receives FDA Approval as a First-in-Class, Twice-Yearly Treatment Option for People Living With Multi-Drug Resistant HIV - (12/22/22)
FDA Product Insert - Sunlenca (lenacapavir) Receives FDA Approval - (12/29/22)
Simulations for once weekly dosing of oral lenacapavir
Summary
Background
Antiretroviral agents with novel mechanisms and dosing intervals could expand treatment options for people with HIV. Lenacapavir, an inhibitor of capsid protein that makes use of a unique mechanism, can be administered orally or subcutaneously. We sought to explore the efficacy of lenacapavir in various combination regimens as initial and maintenance therapy for HIV.
Methods
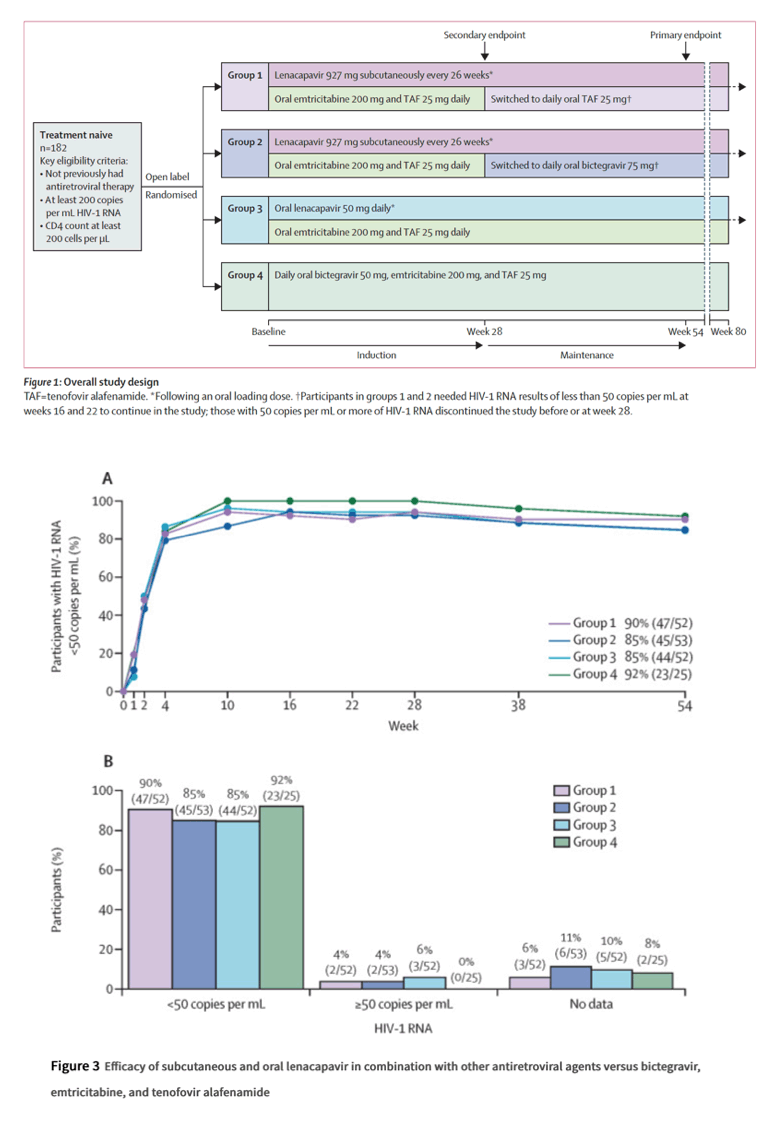
In a phase 2, randomised, open-label, ongoing study at 41 investigational sites in the USA and Dominican Republic, we randomly assigned adults with HIV who had not previously received antiretrovirals to four groups (2:2:2:1). Randomisation was stratified by plasma HIV-1 RNA load (≤100 000 or >100 000 copies per mL) at screening.
Groups 1 and 2 both received lenacapavir (927 mg) subcutaneously every 26 weeks (after 2 weeks of oral loading [600 mg on days 1 and 2, followed by 300 mg on day 8]) with oral daily emtricitabine (200 mg) and tenofovir alafenamide (25 mg) for 28 weeks followed by subcutaneous lenacapavir (927 mg) plus oral daily tenofovir alafenamide (25 mg, group 1) or bictegravir (75 mg, group 2).
Group 3 received oral daily lenacapavir (600 mg on days 1 and 2, followed by 50 mg daily) with emtricitabine (200 mg) and tenofovir alafenamide (25 mg).
Group 4 received oral daily bictegravir (50 mg), emtricitabine (200 mg), and tenofovir alafenamide (25 mg).Participants and investigators were not masked to group assignment. The primary endpoint was the percentage of participants with virological suppression (HIV-1 RNA <50 copies per mL) at week 54, analysed in the full analysis set (all randomly assigned participants who received at least one dose of study drug) using only on-treatment data. The safety outcome measures were incidences of treatment-emergent adverse events and graded laboratory abnormalities, analysed in the full analysis set. This study is registered at ClinicalTrials.gov, NCT04143594.
Findings
Between Nov 22, 2019, and Aug 27, 2020, 249 people with HIV were screened, 183 participants were randomly assigned and 182 received a dose of antiretroviral drugs (52 in group 1, 53 in group 2, 52 in group 3, and 25 in group 4). 22 participants did not complete the full study course (five in group 1, 12 in group 2, four in group 3, and one in group 4).
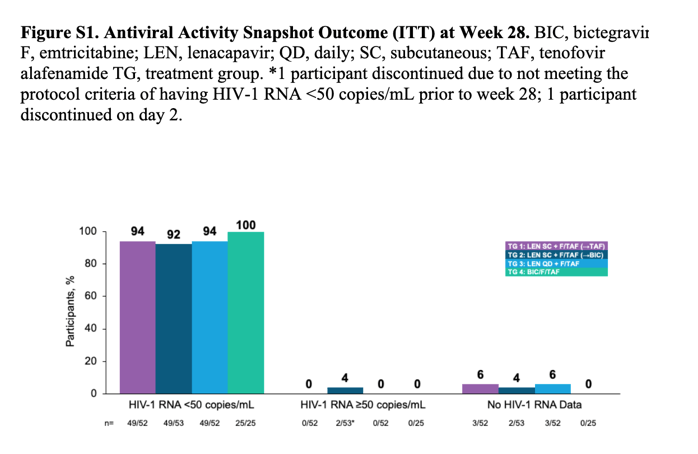
At week 54, virological suppression was 90% (47 of 52 patients) for group 1 (difference vs group 4: -2·6%, 95% CI -18·4 to 13·2), 85% (45 of 53) for group 2 (-7·1%, -23·4 to 9·3), 85% (44 of 52) for group 3 (-7·2%, -23·5 to 9·1), and 92% (23 of 25) for group 4.
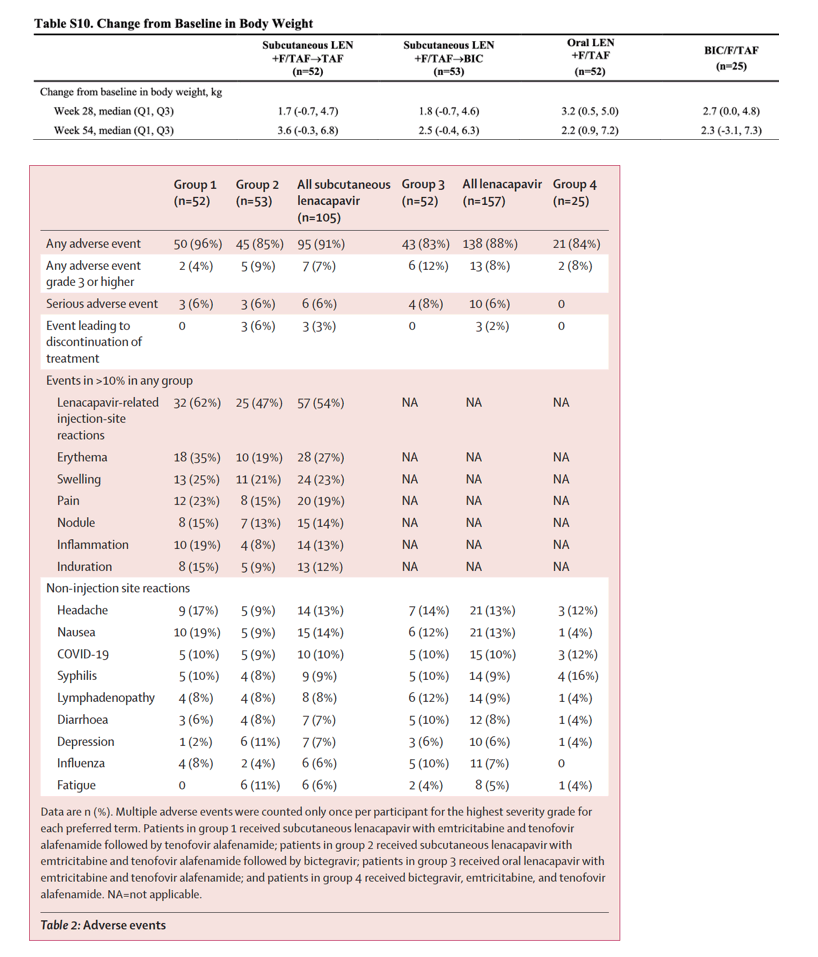
The most frequent non-injection-site adverse events with lenacapavir (subcutaneous or oral) were headache (13%, 21 of 157) and nausea (13%, 21 of 157). The most common lenacapavir-related injection-site reactions were erythema (27%, 28 of 105), swelling (23%, 24 of 105), and pain (19%, 20 of 105), which were generally mild or moderate. No serious adverse event related to study treatment occurred. Three participants discontinued subcutaneous lenacapavir because of grade 1 injection-site reactions (two for induration and one for erythema or swelling).
Interpretation
Lenacapavir warrants further investigation as a potential antiretroviral used orally and as injection in combination with other antiretroviral drugs.
Funding
Gilead Sciences.
Six participants met the protocol-defined virological failure criteria and were tested for resistance: two (2%) of 105 receiving subcutaneous lenacapavir with emtricitabine and tenofovir alafenamide followed by tenofovir alafenamide or bictegravir, three (6%) of 52 receiving oral lenacapavir with emtricitabine and tenofovir alafenamide, and one (4%) of 25 receiving bictegravir, emtricitabine, and tenofovir alafenamide. Four of these six participants had viral rebound, then later resuppressed without changing treatment regimens; none developed resistance. Emergent resistance was found in two participants (one in group 2 and one in group 3). In the participant from group 2, the lenacapavir-associated capsid substitutions Gln67His and Lys70Arg and the Met184Met/Ile reverse transcriptase mutation were detected at week 10 (appendix p 9). Plasma concentrations of lenacapavir were in the target range, and those of emtricitabine and tenofovir were consistent with expected pharmacokinetics. Additional exploratory deep sequencing analyses subsequently detected Met184Ile/Val substitutions in reverse transcriptase at week 2, whereas the Gln67His mutation in capsid was first detected at week 4. 15 In the participant from group 3, virological rebound occurred at week 54 with emergent resistance to lenacapavir (Gln67His; appendix p 9); plasma drug concentrations (lenacapavir and tenofovir alafenamide) suggested inconsistent adherence to all components of the regimen at the time of resistance emergence.
Emergent resistance mutations to lenacapavir were rare; they occurred in two of 157 participants who received lenacapavir, and in these two incidents emergent resistance appeared to be associated with incomplete adherence to oral daily agents. In one participant in group 2 (receiving subcutaneous lenacapavir plus oral daily emtricitabine and tenofovir alafenamide), deep sequencing analyses showed that the emtricitabine resistance mutation Met184Ile/Val emerged before lenacapavir mutations at week 4, which suggests that incomplete adherence to emtricitabine and tenofovir alafenamide preceded emergent lenacapavir resistance. 15 In the other participant, in group 3 (receiving oral daily lenacapavir plus oral daily tenofovir alafenamide), poor adherence to tenofovir alafenamide and lenacapavir was documented by plasma drug concentrations.20
Lenacapavir was generally well tolerated. Most adverse events, including injection-site reactions, were mild or moderate. Discontinuations due to injection-site reactions were infrequent, occurring in only three (of 53) participants receiving subcutaneous lenacapavir. Results of this study, supported by others including subcutaneous lenacapavir,11 indicate that injection-site reactions were not an impediment to the participants in this study.
Three participants discontinued because of an adverse event; all were due to injection-site reactions among participants receiving subcutaneous lenacapavir. Two had grade 1 injection-site induration after the first injection, and one had grade 1 adverse events of injection-site erythema and injection-site swelling after the second injection.
Laboratory abnormalities of grade 3 or higher occurred in 39 (25%) of 156 lenacapavir-treated participants and six (24%) of 25 bictegravir, emtricitabine, and tenofovir alafenamide-treated participants, with no pattern suggesting a specific laboratory abnormality related to lenacapavir (appendix p 19).
Changes in weight from baseline to week 54 were similar for participants receiving lenacapavir (subcutaneous or oral; median change 2·6 kg, IQR 0·2 to 6·8) and participants receiving bictegravir, emtricitabine, and tenofovir alafenamide (median change 2·3 kg, -3·1 to 7·3; appendix p 21).
|
|
| |
| |
|
|
|