| |
Bictegravir/emtricitabine/tenofovir alafenamide plus doravirine in highly treatment-experienced men with multidrug-resistant HIV
|
| |
| |
Download the PDF here
Sterman, Felicia L.a,b; Lalezari, Jacob P.b; Kowalczyk, Ula M.b; Main, David W.c; Grant, Edward M.c; Caro, Luzelenad; Manning, Cassie M.e; Burke, Rochelle L.e
Conclusion
This study found that switching older HTE PWH with MDR virus from a stable regimen of RPV/FTC/TAF plus DTG to BIC/FTC/TAF plus DOR was safe and efficacious, providing a treatment option for this patient population that is compatible with PPIs and H2 blockers, is active against HBV coinfection, has a low pill burden, is well tolerated, and can be taken without food restrictions. Additionally, participants in this study showed no evidence of concerning effects on BMI or QOL measures assessed.
Compared with non-HTE and treatment-naive PWH, HTE PWH are older and have a higher daily pill burden [16]. In addition to extensive antiretroviral treatment histories and high numbers of resistance mutations, participants in this study had a median age of 65 years and most had multiple health conditions and concomitant medications. These factors led their HIV healthcare provider to consider simplified regimens such as DTG/RPV or BIC/FTC/TAF as too risky and unlikely to result in continued virologic suppression.
Many providers proactively switch their aging patients away from older nucleoside reverse transcriptase inhibitors (NRTIs) and protease inhibitors (PIs). This practice preemptively reduces the risk for certain antiretroviral-related DDIs and AEs involving renal, liver, cardiovascular, central nervous system, metabolic, and bone health [5]. Previous studies have established an association between some PI-based regimens and increased risk for cardiovascular events [31-33]. Additionally, expert guidance now recommends switching older PWH with high risk for fragility fractures off of tenofovir disoproxil fumarate (TDF) and/or boosted PIs due to the association of these agents with decreases in bone mineral density relative to regimens containing other NRTIs and INSTIs [5,34,35]. There is a continued need for establishing the safety and efficacy of regimens that do not contain a PI, booster, or TDF in aging PWH.
In general, selecting an efficacious and well tolerated antiretroviral regimen for older PWH can be challenging. Age-related decreases in renal and liver function influence choice of antiretroviral medications, and potential interactions between antiretroviral medications and drugs used to manage comorbidities must be considered [5]. A study of PWH in France found that 62% of older patients whose HIV was diagnosed before 2000 had one or more comorbidities, and 71% were receiving at least 1 co-medication [28]. In a cross-sectional study in PWH aged ≥65 years, the prevalence of comorbidities and polypharmacy increased with both older age and longer duration of HIV infection; those infected for 10 or more years had a higher probability of comorbidities compared with HIV-uninfected controls, and independent predictors for the presence of comorbidities included age ≥75 years, male gender, and HIV duration above 20 years [29]. There is also evidence that PWH ≥50 years old are more likely to be prescribed an antiretroviral/non-antiretroviral combination that is either explicitly contraindicated or known to have moderate to high evidence of interaction relative to PWH <50 years [30]. Accordingly, an antiretroviral regimen with a low potential for DDIs may provide significant advantages in aging PWH. As BIC/FTC/TAF plus DOR does not interact with PPIs or H2 blockers, it could be a beneficial regimen in older populations of HTE PWH with MDR virus.
Abstract
Objective:
To evaluate the safety and efficacy of switching highly treatment-experienced people with HIV (HTE PWH) from rilpivirine/emtricitabine/tenofovir alafenamide (RPV/FTC/TAF) plus dolutegravir (DTG) to bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) plus doravirine (DOR). A pharmacokinetic (PK) analysis was conducted to assess the potential interaction between BIC and DOR.
Design and methods:
This open-label switch trial enrolled HTE PWH from a primary care private practice in the United States. Eligible participants were male, aged ≥45 years, with documented viral resistance to protease inhibitors, nucleoside reverse transcriptase inhibitors, and/or nonnucleoside reverse transcriptase inhibitors but no resistance to RPV or DOR, and no K65R or T69 insertion mutations. Virologic suppression (≤50 copies/ml) while on RPV/FTC/TAF plus DTG for ≥6 months was required prior to enrollment. The primary endpoint of the study was virologic suppression (<50 and <200 copies/ml) at 48 weeks. Secondary endpoints included safety, tolerability, changes in body mass index (BMI), and identification of PK parameters of BIC and DOR.
Results:
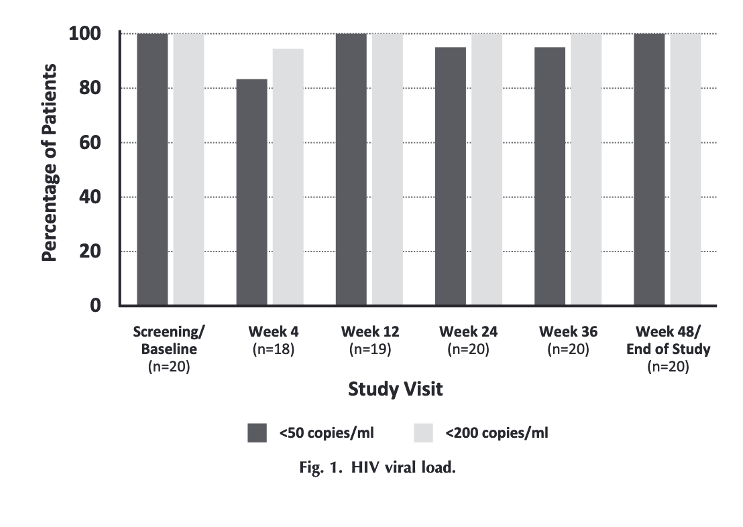
Twenty males [median age: 65 years (range, 46-74), median time since HIV diagnosis: 37 years (range, 12-42)] completed the study. BIC/FTC/TAF plus DOR was well tolerated with no serious or treatment-related adverse events reported and no appreciable changes in BMI from baseline to Week 48. At Week 48, 100% of participants had <50 viral copies/ml. PK parameters for BIC and DOR (n = 10) were consistent with published data.
Conclusions:
Switching from RPV/FTC/TAF plus DTG to BIC/FTC/TAF plus DOR was well tolerated and efficacious in HTE men aged ≥45 years with HIV.
|
|
| |
| |
|
|
|