| |
Long-acting Injectable Cabotegravir/Rilpivirine Effective in a Small Patient Cohort With Virologic Failure on Oral Antiretroviral Therapy
|
| |
| |
Download the PDF
"this cohort further supports the use of iCAB/RPV in patients with viremia."
In summary, this cohort further supports the use of iCAB/RPV in patients with viremia. This regimen has been utilized in our practice as a salvage option for people with long-standing viremia and often advanced disease, where the potential benefits of virologic suppression far outweigh the risks of treatment emergent resistance. Directly observed therapy through Q2M injections provides a much-needed therapeutic option for thousands of people in the United States who have not achieved virologic suppression. Although this cohort has not demonstrated virologic failure to date, there was a 4-fold increase in virologic failure in Q2M dosing compared to Q1M dosing in the ATLAS 2M trial (8/522 vs 2/523), and the convenience of direct-to-Q2M dosing in salvage should be weighed against a concern for higher potential for virologic failure and development of further resistance-associated mutations. Up to 10% of the ASCC patient cohort has not achieved virologic suppression at any time point with durable virologic suppression even more difficult to maintain. This is despite intensive case management and social support services offered to our patients in a resource-rich clinical setting. Unfortunately, rilpivirine- and cabotegravir-associated mutations are common in the cohort of patients in our practice with inconsistent or no virologic suppression. Reassuringly, 3 patients with minor INSTI mutations with minimal effect on CAB achieved virologic suppression with follow-up data up to 1 year for 3 patients. Long-term outcomes of this growing cohort will continue to be collected and reported as experience with this treatment strategy accumulates, and additional long-acting ART formulations are greatly needed to expand the toolkit in the effort to reach 100% virologic suppression.
Implementation of this program has required close collaboration of clinicians, case management, and pharmacists. iCAB/RPV has been included in the Mississippi Medicaid preferred drug list without viral suppression restrictions.
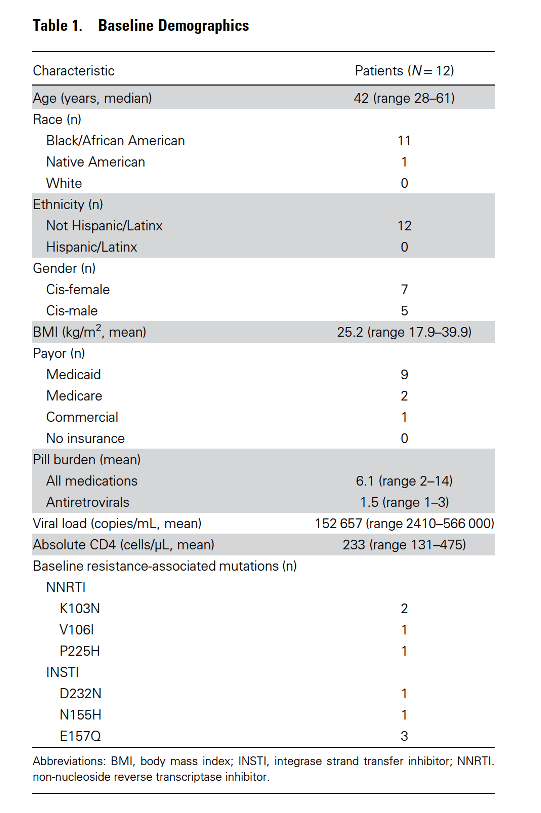
Baseline historical resistance-associated mutations were common, present in 83% (10 of 12) of patients.
Three patients had substance use disorder, including alcohol, methamphetamine, and cocaine. No patients reported injection drug use. No patients had hepatitis B coinfection. One patient had housing instability. Three patients had major depressive disorder, and 1 patient had paranoid schizophrenia. Before starting iCAB/RPV, the mean number of total pills prescribed was 6.1 (range 2-14), and the mean number of ART pills was 1.5 (range 1-3).
Virologic outcomes of this cohort were excellent. The mean viral load was 152 657 copies/mL (range 2410-566 000) at baseline. All patients achieved viral load <50 copies/mL within 3 months of initiation of iCAB/RPV, and virologic rebound (viral load ≥200 copies/mL) has not been observed to date.
Immunologic outcomes in this cohort were positive in all but 1 participant. At baseline, 5 patients had a current AIDS diagnosis with prior diagnoses of Candida esophagitis (3), cryptococcal meningitis (1), and Pneumocystispneumonia (1). Baseline absolute CD4 count mean was 233 cells/μL (range 131-475). Mean CD4 increase was 184 cells/μL (range 26-414), and patients with CD4 <200 cells/μL at baseline had a mean absolute CD4 increase of 308% (range 64%-844%) during this short follow-up period. Among the 3 patients with evidence of wasting at baseline, weight gain ranged from 2.6 to 6.9 kg during follow-up.
|
|
| |
| |
|
|
|