| |
Risk of Gastrointestinal Adverse Events Associated
With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss
|
| |
| |
JAMA. October 5, 2023
------------------------------------------
Novo Nordisk's weight-loss drugs pose small risk of severe stomach complications: study
oct 6 2023
https://www.fiercepharma.com/pharma/novo-nordisks-ozempic-wegovy-pose-added-risk-stomach-disorders-weight-loss-patients-study
The first-of-it-kind investigation indicates a link between Novo’s popular GLP-1 products and the gastrointestinal conditions gastroparesis, pancreatitis and bowel obstruction. In the report, the investigators emphasized that the risk was very small.
Compared to patients on Contrave, those on the GLP-1 drugs were 9.1 times more likely to have pancreatitis, 4.2 times more likely to have a bowel obstruction and 3.7 times more likely to develop gastroparesis, the study found.
Compared to patients on Contrave, those on the GLP-1 drugs were 9.1 times more likely to have pancreatitis, 4.2 times more likely to have a bowel obstruction and 3.7 times more likely to develop gastroparesis, the study found.
Popular weight loss drugs linked to rare but severe stomach problems, study finds (study full text below)
Oct. 5, 2023,
https://www.nbcnews.com/health/health-news/ozempic-wegovy-linked-severe-medical-conditions-stomach-problems-study-rcna118823
Pancreatitis, or inflammation of the pancreatitis, occurred at a rate of about 5 cases per 1,000 users of semaglutide and 8 cases per 1,000 users of liraglutide. The condition causes severe abdominal pain and, in some cases, requires hospitalization and surgery.
Gastroparesis was seen at a rate of about 10 cases per 1,000 semaglutide users and 7 cases per 1,000 liraglutide users. The condition, which can be difficult to treat, causes severe nausea, vomiting and abdominal pain.
They took blockbuster drugs for weight loss and diabetes. Now their stomachs are paralyzed
July 25, 2023
https://www.cnn.com/2023/07/25/health/weight-loss-diabetes-drugs-gastroparesis/index.html
Facial lipoatrophy with semaglutide-related weight loss
February 10, 2023
https://www.mdedge.com/dermatology/article/261159/aesthetic-dermatology/facial-lipoatrophy-semaglutide-related-weight-loss
---------------------------------------
Glucagon-like peptide 1 (GLP-1) agonists are medications approved for treatment of diabetes that recently have also been used off label for weight loss.1 Studies have found increased risks of gastrointestinal adverse events (biliary disease,2 pancreatitis,3 bowel obstruction,4 and gastroparesis5) in patients with diabetes.2-5 Because such patients have higher baseline risk for gastrointestinal adverse events, risk in patients taking these drugs for other indications may differ. Randomized trials examining efficacy of GLP-1 agonists for weight loss were not designed to capture these events2 due to small sample sizes and short follow-up. We examined gastrointestinal adverse events associated with GLP-1 agonists used for weight loss in a clinical setting.
Results
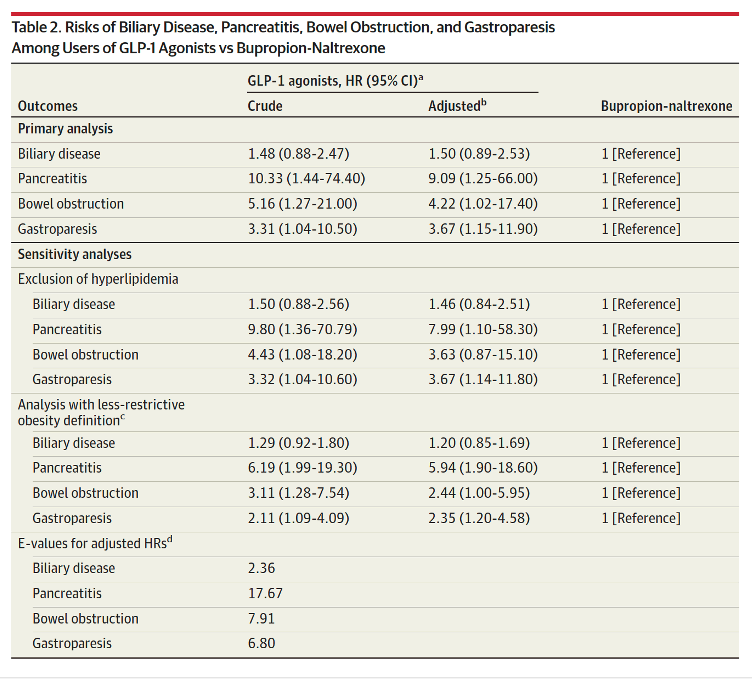
Our cohort included 4144 liraglutide, 613 semaglutide, and 654 bupropion-naltrexone users. Incidence rates for the 4 outcomes were elevated among GLP-1 agonists compared with bupropion-naltrexone users (Table 1). For example, incidence of biliary disease (per 1000 person-years) was 11.7 for semaglutide, 18.6 for liraglutide, and 12.6 for bupropion-naltrexone and 4.6, 7.9, and 1.0, respectively, for pancreatitis.
Use of GLP-1 agonists compared with bupropion-naltrexone was associated with increased risk of pancreatitis (adjusted HR, 9.09 [95% CI, 1.25-66.00]), bowel obstruction (HR, 4.22 [95% CI, 1.02-17.40]), and gastroparesis (HR, 3.67 [95% CI, 1.15-11.90) but not biliary disease (HR, 1.50 [95% CI, 0.89-2.53]). Exclusion of hyperlipidemia from the analysis did not change the results (Table 2). Inclusion of GLP-1 agonists regardless of history of obesity reduced HRs and narrowed CIs but did not change the significance of the results (Table 2). E-value HRs did not suggest potential confounding by BMI.
Discussion
This study found that use of GLP-1 agonists for weight loss compared with use of bupropion-naltrexone was associated with increased risk of pancreatitis, gastroparesis, and bowel obstruction but not biliary disease.
Given the wide use of these drugs, these adverse events, although rare, must be considered by patients who are contemplating using the drugs for weight loss because the risk-benefit calculus for this group might differ from that of those who use them for diabetes.
Limitations include that although all GLP-1 agonist users had a record for obesity without diabetes, whether GLP-1 agonists were all used for weight loss is uncertain.
|
|
| |
| |
|
|
|