| |
HIV-1 drug resistance in people on dolutegravir-based antiretroviral therapy: a collaborative cohort analysis
|
| |
| |
Download the PDF here
Download the PDF here
In conclusion, our study underlines the importance of resistance testing, especially in treatment-experienced people. Although rare, dolutegravir resistance can develop in people who experience viraemia on a dolutegravir-containing ART regimen. Monitoring the emergence of such resistance is important to prevent resistance at the individual and the population level and to ensure the long-term sustainability of ART.
Dolutegravir has a high genetic barrier to resistance,3, 4 and relatively few people living with HIV are so far known to have developed resistance.5, 6, 7.
The risk factors and the mutational patterns that confer resistance to dolutegravir in vivo are less well established than for older antiretroviral drugs.16
The widespread use of dolutegravir in resource-limited settings, where ART regimens are highly standardised, drugs are recycled, access to adherence support and viral load and resistance testing is limited, and the risk for drug stock-outs is higher, might facilitate the emergence of resistance. In the DTG RESIST study, we combined data from European, North American, and South African cohorts to identify risk factors for dolutegravir resistance and examine the patterns of resistance mutations across different HIV-1 subtypes.
------------------------------------
COMMENTARY:Several observations emerge from this important paper. First and foremost, although not directly addressed in it, viral failure on second-generation generation INSTI-based regimens is uncommon, reinforcing the advantage of global access to these highly effective antiretrovirals. As we investigate resistance, we should not lose focus on this major success and its high impact on people's lives.
Second, among those in whom therapy failed, although dolutegravir resistance was rare, it existed, and in similar proportions to the highest levels from clinical trials, representing a concerning trend.8
Moreover, the higher frequency in people taking dual-therapy as compared with triple-therapy regimens is also worrying, as these regimens are increasingly used for treatment initiation and optimisation. Lastly, the increased dolutegravir resistance with NRTI resistance is particularly concerning, considering that extrapolation of data from clinical trials evaluating optimal regimens after failure of first-line therapy might lead to a questionable perception that there is little necessity for or benefit from resistance testing, due to potential diminished relevance of NRTI resistance.9
Third, several outstanding issues exist. Most data are from people with HIV-1 subtype B, whereas non-B subtypes predominate globally. Access to individual viral load and resistance monitoring is limited in many low and middle income and high-burden settings. And global roll-out of dolutegravir-based regimens in all therapy lines is still ongoing, and the parallel use of long-acting antiretrovirals for treatment and prevention (eg, cabotegravir) is in its infancy.
Taken together, as the essential global roll-out of dolutegravir-based therapy is expanded, data presented by Loosli and colleagues suggest that now might be a good time to also notice that dolutegravir resistance can and does occur and could potentially be even higher than anticipated. We should prepare for monitoring and minimising resistance, so that we do not fall behind in the race. The WHO HIV Drug Resistance Network is appropriately planning on doing exactly that on a population level; we should also consider making plans to expand this for individual care.10
---------------------------
Background
The widespread use of the integrase strand transfer inhibitor (INSTI) dolutegravir in first-line and second-line antiretroviral therapy (ART) might facilitate emerging resistance. The DTG RESIST study combined data from HIV cohorts to examine patterns of drug resistance mutations (DRMs) and identify risk factors for dolutegravir resistance.
Methods
We included cohorts with INSTI resistance data from two collaborations (ART Cohort Collaboration, International epidemiology Databases to Evaluate AIDS in Southern Africa), and the UK Collaborative HIV Cohort. Eight cohorts from Canada, France, Germany, Italy, the Netherlands, Switzerland, South Africa, and the UK contributed data on individuals who were viraemic on dolutegravir-based ART and underwent genotypic resistance testing. Individuals with unknown dolutegravir initiation date were excluded. Resistance levels were categorised using the Stanford algorithm. We identified risk factors for resistance using mixed-effects ordinal logistic regression models.
Findings
We included 599 people with genotypic resistance testing on dolutegravir-based ART between May 22, 2013, and Dec 20, 2021. Most had HIV-1 subtype B (n=351, 59%), a third had been exposed to first-generation INSTIs (n=193, 32%), 70 (12%) were on dolutegravir dual therapy, and 18 (3%) were on dolutegravir monotherapy.
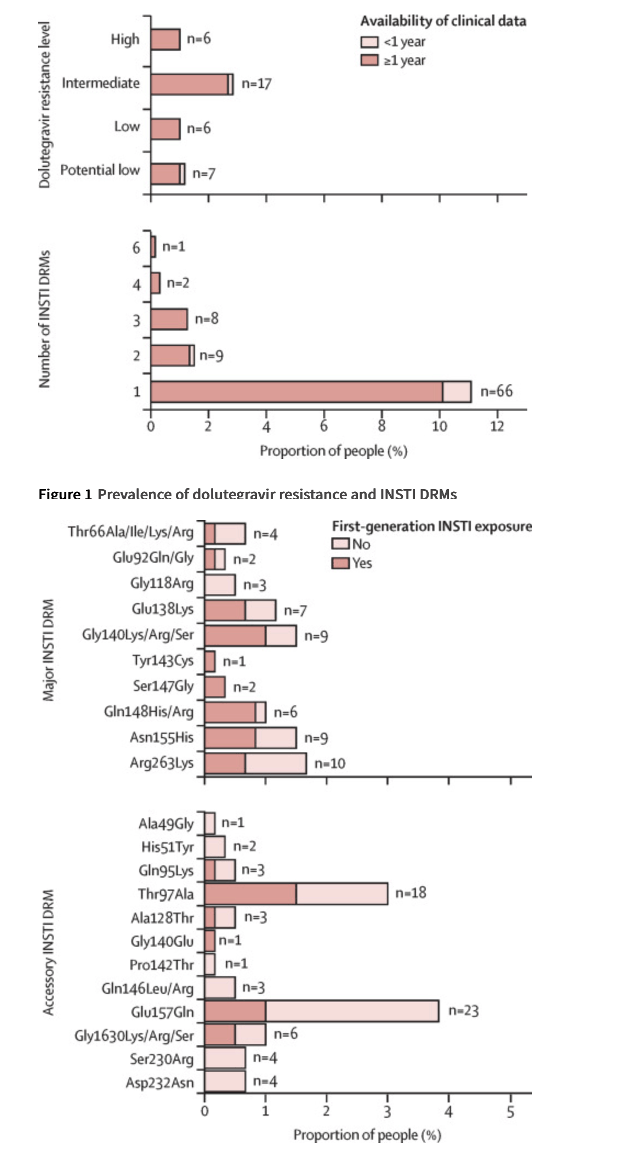
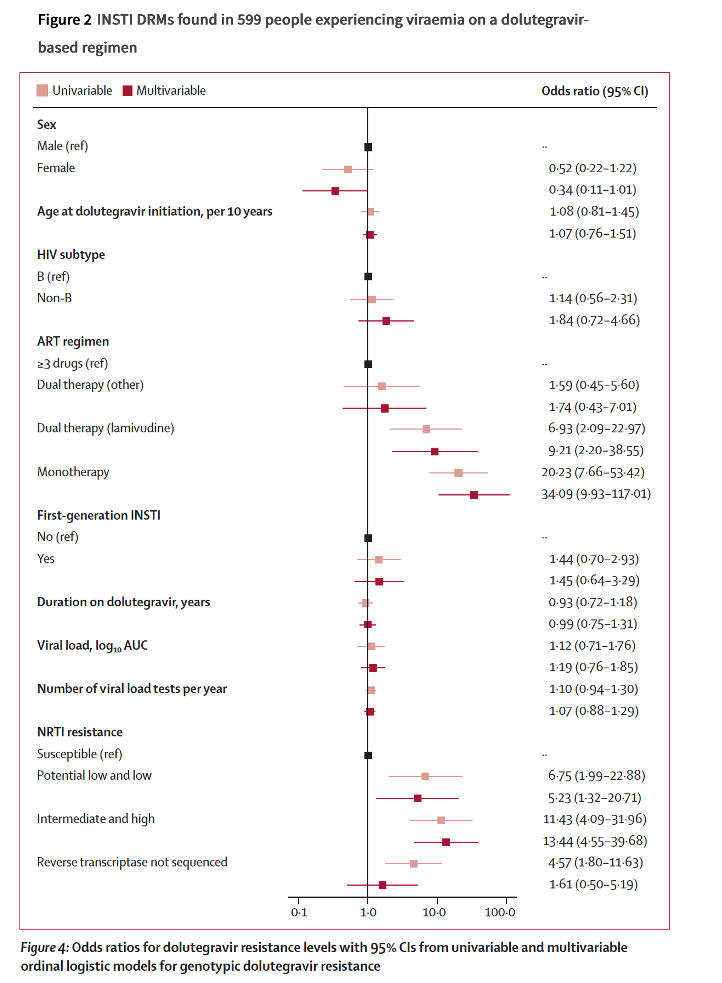
INSTI DRMs were detected in 86 (14%) individuals; 20 (3%) had more than one mutation. Most (n=563, 94%) were susceptible to dolutegravir, seven (1%) had potential low, six (1%) low, 17 (3%) intermediate, and six (1%) high-level dolutegravir resistance. The risk of dolutegravir resistance was higher on dolutegravir monotherapy (adjusted odds ratio [aOR] 34⋅1, 95% CI 9⋅93–117) and dolutegravir plus lamivudine dual therapy (aOR 9⋅21, 2⋅20–38⋅6) compared with combination ART, and in the presence of potential low or low (aOR 5⋅23, 1⋅32–20⋅7) or intermediate or high-level (aOR 13⋅4, 4⋅55–39⋅7) nucleoside reverse transcriptase inhibitor (NRTI) resistance.
About a third of participants had previously been exposed to first-generation INSTIs; most were exposed to raltegravir (142/193), followed by elvitegravir (38/193), and both elvitegravir and raltegravir (13/193). A total of 129 participants did not have a CD4 measurement within a year of the genotypic resistance test, ten did not have any recorded HIV-1 RNA measurements before the genotypic resistance test, and in 62 people sequencing did not cover reverse transcriptase.
Interpretation
Among people with viraemia on dolutegravir-based ART, INSTI DRMs and dolutegravir resistance were rare. NRTI resistance substantially increased the risk of dolutegravir resistance, which is of concern, notably in resource-limited settings. Monitoring is important to prevent resistance at the individual and population level and ensure the long-term sustainability of ART.
|
|
| |
| |
|
|
|