| |
Efficacy, safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir for HIV pre-exposure prophylaxis in transgender women: a secondary analysis of the HPTN 083 trial
|
| |
| |
Download the PDF here
Download the PDF here
Sept 2023
Results from this study specific to transgender women are consistent with the overall HPTN 083 study result, which found that injectable cabotegravir administered intramuscularly every 8 weeks was well tolerated, safe, and highly effective in preventing HIV infection in men who have sex with men and transgender women at substantial risk for acquiring HIV.13
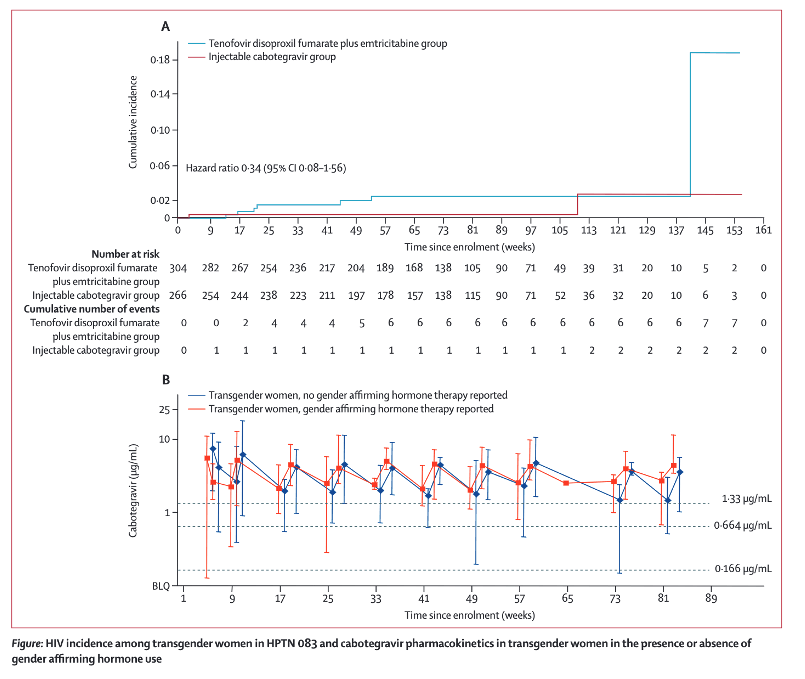
Cabotegravir concentrations were not statistically different between transgender women who reported using gender affirming hormone therapy compared with transgender women who reported not using this therapy. However, additional studies, which capture detailed gender affirming hormone therapy dosing information and hormone concentrations, are needed to more fully characterise the relationship between cabotegravir and these therapies.
Nine seroconversions occurred among transgender women during the blinded phase of the study (seven in the tenofovir disoproxil fumarate plus emtricitabine group and two in the injectable cabotegravir group); overall incidence was 1⋅19 per 100 person-years (95% CI 0⋅54-2⋅25): 1⋅80 per 100 person-years (0⋅73-3⋅72) in the tenofovir disoproxil fumarate plus emtricitabine group and 0⋅54 per 100 person-years (0⋅07-1⋅95) in the injectable cabotegravir group (hazard ratio 0⋅34 [95% CI 0⋅08-1⋅56]). Cabotegravir concentrations did not differ by gender affirming hormone therapy use.
During the blinded phase of the trial, no transgender women participants who received on-time cabotegravir injections acquired HIV. However, one transgender woman in the injectable cabotegravir group acquired HIV during the oral phase of the study. The participant acquired HIV 27 days after enrolment (cabotegravir concentration at first HIV-positive visit: 6⋅30 μg/mL), and had a cabotegravir concentration 8 × PA-IC90 or greater at the preceding visit. Although pill counts were used to assess adherence during the trial, direct observation was not performed; thus, it is unclear if the participant used oral cabotegravir consistently. It is also possible that the participant acquired HIV shortly after enrolment and that this was not detected until the week 4 visit.
---------------------------------------
Commentary: In The Lancet HIV, Mark Marzinke and colleagues7 report a secondary analysis from the HIV Preventions Trials Network (HPTN) 083 trial of injectable cabotegravir, focused on the 570 participants enrolled under the umbrella of transgender women. Although the sample size was too small to achieve statistical significance, the magnitude and direction of effect were in line with the results from the overall trial population. Gender identity was only captured at enrolment and the authors recommend that this be assessed longitudinally in future trials as they observed fluidity in this demographic variable, with self-identified men who have sex with men reporting gender affirming hormonal therapy at enrolment and during follow-up. There were no differences in cabotegravir concentrations between transgender women reporting using gender affirming hormone therapy and those who did not, which is reassuring. However, additional pharmacological studies capturing the details of dosing are needed to fully assess drug-drug interactions.
Adherence in the injectable cabotegravir treatment group was high, with 92% of both transgender women and men who have sex with men receiving an injection within 2 weeks of the prescribed schedule. Adherence to tenofovir disoproxil fumarate plus emtricitabine was assessed in a random subset of participants and found to be significantly lower in transgender women than in men who have sex with men. Nonetheless, 58% had drug concentrations compatible with four or more doses of tenofovir disoproxil fumarate plus emtricitabine per week, which is more than twice that observed in preceding studies 5
in similar populations. Even though injectable cabotegravir was much more effective, this improvement in adherence to tenofovir disoproxil fumarate plus emtricitabine is encouraging. We think the reasons for this improvement are multifactorial, including greater awareness and confidence in PrEP effectiveness in the communities. Marzinke and colleagues note the need to place PrEP in a sociobehavioural context that resonates with transgender women if PrEP uptake is to improve in this community.
While the implementation evidence is being gathered for injectable cabotegravir, we must make the best of tenofovir disoproxil fumarate plus emtricitabine and generics.
As such, WHO's 2022 technical brief on differentiated and simplified PrEP for HIV prevention9 is most welcome. The brief embraces self-testing as an additional choice for PrEP users and provides clear guidance on starting and stopping oral PrEP for two categories of population. Cisgender men, transgender, and gender diverse populations assigned male at birth who have sexual exposure and are not taking exogenous estradiol-based hormones are in one category. They can start with a double dose (two tablets) 2-24 h before sex and stop after 2 days of single tablets (the on-demand regimen). Everyone else is in the second category, for whom a 7-day start and 7-day stop are recommended. The level of evidence supporting the double dose start is available from randomised placebo-controlled trials for the first category, but not for the second category, as the trials have only evaluated daily oral PrEP. However, there have been well designed pharmacological studies characterising PrEP concentrations within tissue sites that facilitate modelling population effectiveness and map well to clinical trial effectiveness.10
There is consistent support that a tenofovir disoproxil fumarate plus emtricitabine double-dose (two tablets) will achieve population effective drug concentrations within 24 h in the peripheral blood, female genital tract, and colorectal tissues. It is important for PrEP providers to ensure that PrEP users in the second category described above are educated about a double-dose start since it is not always possible to initiate daily PrEP 7 days before exposure.
--------------------------------
Summary
Background
The HIV Prevention Trials Network (HPTN) 083 trial showed that long-acting injectable cabotegravir was more effective than tenofovir disoproxil fumarate plus emtricitabine in preventing HIV in cisgender men and transgender women who have sex with men. We aimed to characterise the cohort of transgender women included in HPTN 083.
Methods
HPTN 083 is an ongoing, phase 2b/3, randomised, multicentre, double-blind, double-dummy clinical trial done at 43 sites in seven countries (Argentina, Brazil, Peru, the USA, South Africa, Thailand, and Viet Nam). HIV-negative participants were randomly assigned (1:1) to receive injectable cabotegravir or tenofovir disoproxil fumarate plus emtricitabine. The study design and primary outcomes of the blinded phase of HPTN 083 have already been reported. An enrolment minimum of 10% transgender women was set for the trial. Here we characterise the cohort of transgender women enrolled from Dec 6, 2016, to May 14, 2020, when the study was unblinded. We report sociodemographic characteristics, use of gender affirming hormone therapy, and behavioural assessments of the transgender women participants. Laboratory testing and safety evaluations are also reported. The trial is registered at ClinicalTrials.gov, NCT02720094.
Findings
HPTN 083 enrolled 570 transgender women (304 tenofovir disoproxil fumarate plus emtricitabine; 266 injectable cabotegravir). Transgender women were primarily from Asia (225 [39%]) and Latin America (205 [36%]); 330 (58%) reported using gender affirming hormone therapy. Intimate partner violence was common (270 [47%] reported emotional abuse and 172 [30%] reported physical abuse) and 323 (57%) reported a history of childhood sexual abuse.
159 (28%) transgender women disagreed that they were at risk for HIV, and 142 (25%) screened positive for depressive symptoms. During study follow-up, incidence of syphilis was 16⋅25% (95% CI 13⋅28-19⋅69), rectal gonorrhoea was 11⋅66% (9⋅14-14⋅66), and chlamydia was 20⋅61% (17⋅20-24⋅49). Frequency of adverse events was similar between the treatment groups.
Nine seroconversions occurred among transgender women during the blinded phase of the study (seven in the tenofovir disoproxil fumarate plus emtricitabine group and two in the injectable cabotegravir group); overall incidence was 1⋅19 per 100 person-years (95% CI 0⋅54-2⋅25): 1⋅80 per 100 person-years (0⋅73-3⋅72) in the tenofovir disoproxil fumarate plus emtricitabine group and 0⋅54 per 100 person-years (0⋅07-1⋅95) in the injectable cabotegravir group (hazard ratio 0⋅34 [95% CI 0⋅08-1⋅56]). Cabotegravir concentrations did not differ by gender affirming hormone therapy use.
Interpretation
HIV prevention strategies for transgender women cannot be addressed separately from social and structural vulnerabilities. Transgender women were well represented in HPTN 083 and should continue to be prioritised in HIV prevention studies. Our results suggest that injectable cabotegravir is a safe and effective pre-exposure prophylaxis option for transgender women.
Funding
National Institute of Allergy and Infectious Diseases and ViiV Healthcare.
|
|
| |
| |
|
|
|