| |
Doravirine Plus Integrase Strand Transfer Inhibitors as a 2-Drug
Treatment-Switch Strategy in People Living with HIV:
The Real-Life DORINI Multicentric Cohort Study
|
| |
| |
Download the PDF here
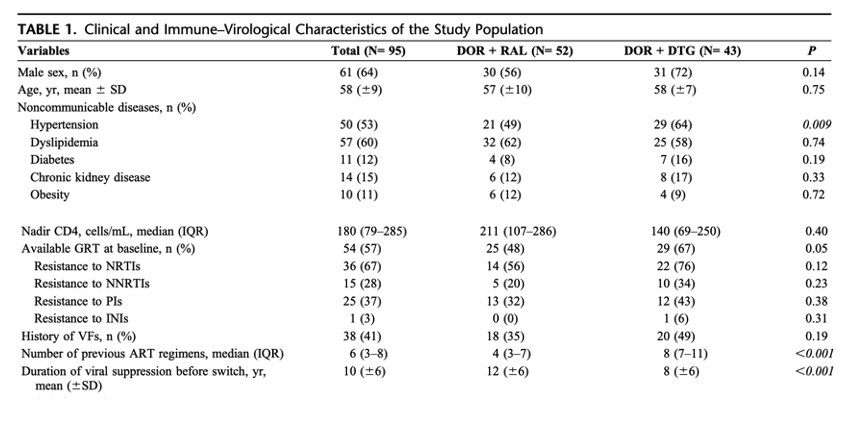
Hypertension was observed in 53% of patients, whereas dyslipidemia was reported in 60%. In addition, a history of hepatitis C virus (HCV) coinfection was reported in 34% of the patients. The most frequent reason to initiate a DOR-containing regimen was to prevent metabolic complications or toxicity issues (proactive switch, 86%). The primary demographics and clinical characteristics of our cohort are summarized in Table 1.
The availability of potent and safer antiretroviral therapy (ART) has opened up the possibility of using two-drug antiretroviral regimens as a switch option for ART-experienced people living with HIV (PLWH). This approach offers several advantages, including cost reduction, decreased drug-drug interactions (DDIs), prevention of ART-related toxicities, and improved adherence to lifelong treatment.1,2
Furthermore, DOR taken as a single agent represents a valuable treatment option for aging patients with issues of polypharmacy and multimorbidity.7 Finally, DOR shows promising efficacy in controlling HIV viral replication even in the presence of major acquired resistance mutations (RAMs) selected by other NNRTIs,8,9 has long pharmacokinetic forgiveness exceeding 72 hours,10 and does not require food restrictions. These features could be crucial in patients with failed NNRTIs or uncertain adherence. Even if not recommended and mentioned by guidelines, DOR could be a good companion of ARV drugs with a high genetic barrier against HIV, such as integrase strand transfer Inhibitors (INIs).11
ABSTRACT
Background:
Few data are available about the efficacy, durability, and tolerability of doravirine (DOR) + integrase strand inhibitors (INI) as a switching strategy among antiretroviral therapy (ART)-experienced people living with HIV (PLWH).
Setting:
Retrospective, multicenter cohort study investigating the durability, efficacy, and tolerability of 2 off-label drug associations of DOR + INI among ART-experienced PLWH.
Methods:
The study included PLWH who switched to DOR combined with either raltegravir (RAL) or dolutegravir (DTG) between June 1, 2020, and December 31, 2021, with at least 1 follow-up (FU) visit. Virologic, biometric, and metabolic parameters were evaluated at baseline (T0) and at 1-3 (T1), 6 (T2), and 12 (T3) months. Univariate and multivariate survival analyses assessed the 28-week probability of persistence on the regimens. Patient satisfaction was measured using the HIV Treatment Satisfaction Questionnaire.
Results:
Ninety-five PLWH were included, 52 in DOR + RAL and 43 in DOR + DTG. Six treatment discontinuations were reported during a mean of 37 (±17) weeks of FU (incidence of 2.7 x 1000 person-weeks FU). Only 2 were the result of virological failure without resistance mutations. DOR + DTG demonstrated significantly higher 28-week persistence than DOR + RAL (HR 1.90, 95% CI: 1.24-2.90, log-rank: P = 0.003). Weight, waist circumference, and fasting lipids reduced considerably at T3 vs T0. Overall, high satisfaction with the new treatment was reported, particularly in the DOR + RAL (68 (64-72)/72), compared with the DOR + DTG group (58 (50-65)/72, P< 0.001).
Conclusions:
Our experience revealed few treatment discontinuations, improved metabolic parameters, and high patient satisfaction among ART-experienced PLWH switching to DOR combined with INI, irrespective of the specific INI used.
|
|
| |
| |
|
|
|