| |
Impact of Treatment Adherence on Efficacy of Dolutegravir + Lamivudine and Dolutegravir + Tenofovir Disoproxil Fumarate/Emtricitabine: Pooled Week 144 Analysis of the GEMINI-1 and GEMINI-2 Clinical Studies
|
| |
| |
Download the PDF here
Nov 2023
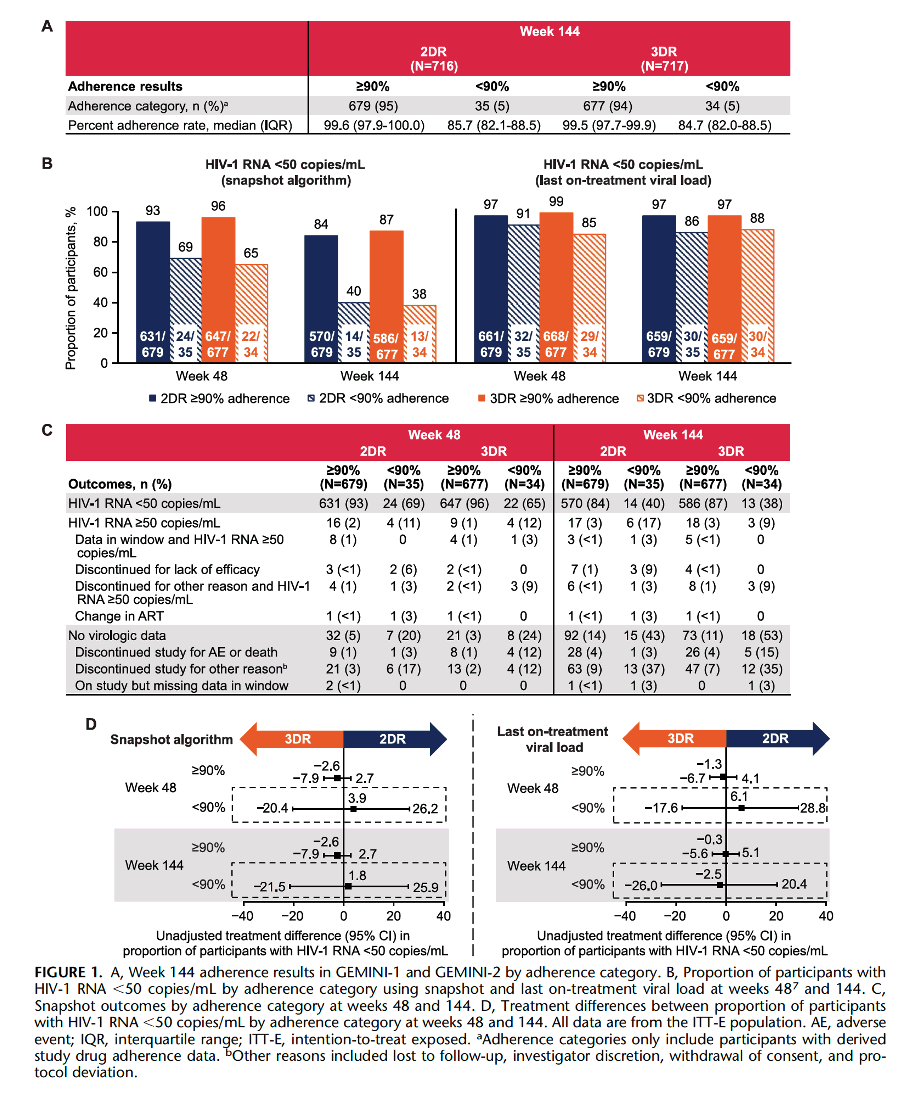
In conclusion, lower adherence was associated with lower rates of virologic suppression at week 144 regardless of treatment group, consistent with 48-week findings.7 These results suggest that the 2DR dolutegravir + lamivudine has similar forgiveness with imperfect adherence as the 3DR dolutegravir + tenofovir disoproxil fumarate/emtricitabine; however, clinicians should continue to advocate for optimal adherence (ie, "every dose, every day") to support people living with HIV-1 in achieving and maintaining virologic suppression to decrease the risks of virologic rebound, HIV-1 transmission, and resistance development.
Similar rates of virologic suppression were observed between 2-drug and 3-drug dolutegravir-based regimens regardless of treatment adherence category through 3 years of treatment in the GEMINI-1 and GEMINI-2 trials. As expected, virologic suppression rates were lower when adherence was <90% vs ≥90% for both treatment groups. As observed at week 48, virologic response rates in each adherence category were high by last on-treatment viral load analysis regardless of treatment regimen at week 144; however, slightly lower rates were observed in those with <90% vs ≥90% adherence. Response rates in the <90% adherence category for both treatment groups were higher in last on-treatment viral load vs snapshot analyses, with the difference mostly driven by nonvirologic reasons such as lost to follow-up and withdrawal of consent. Unlike in vitro findings that suggest that different integrase inhibitor–based regimens have varying levels of regimen forgiveness,8 clinical evidence from this study indicates that lower adherence reduces virologic efficacy to the same extent regardless of regimen and highlights the importance of interpreting in vitro data with caution, especially when superseded by clinical data. This is consistent with the randomized controlled GS-US-380-1489 trial comparing the 3DRs bictegravir/tenofovir alafenamide/emtricitabine with dolutegravir/abacavir/lamivudine, which reported similar efficacy outcomes among participants using more stringent ≥95% or <95% adherence thresholds assessed using pill count.9
The unadjusted difference in proportion of participants who achieved virologic suppression at week 144 between the 2DR and 3DR treatment groups was similar based on snapshot analysis and last on-treatment viral load (Fig. 1D).
|
|
| |
| |
|
|
|