 |
 |
 |
| |
High rates of long-term HIV RNA re-suppression
after virological failure on dolutegravir in the ADVANCE trial.
|
| |
| |
Regaining HIV Control More Likely With DTG Than EFV in ADVANCE
IAS 2023, July 23-26, 2023. Brisbane
Mark Mascolini
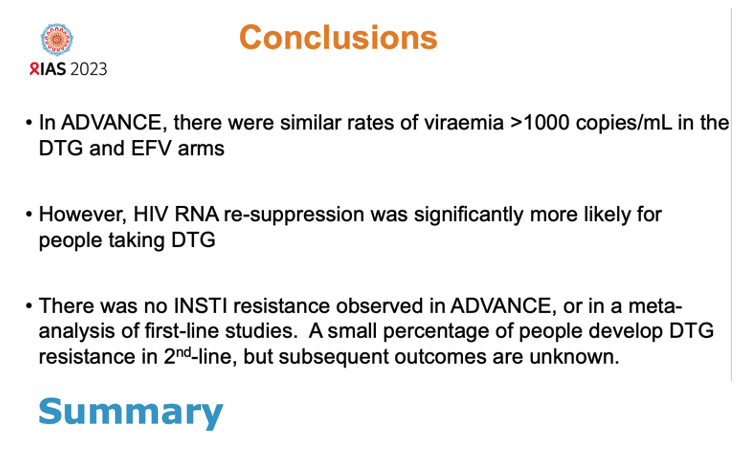
Antiretroviral regimens (ART) built on dolutegravir (DTG) or efavirenz (EFV) had similar virologic failure rates in the South African ADVANCE trial [1]. But renewed control of HIV proved more likely in people randomized to DTG than to EFV (95% vs 66% through 48 weeks). No resistance to the HIV integrase inhibitor class-of which DTG is a member-arose during 192 weeks in ADVANCE.
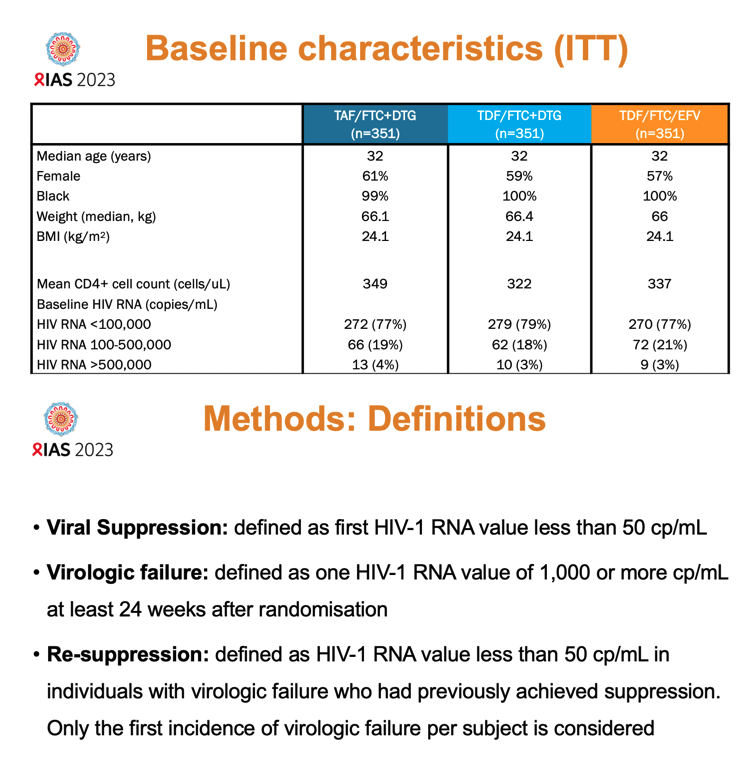
ADVANCE randomized 1053 South Africans to DTG plus emtricitabine (FTC) and either tenofovir alafenamide (TAF) or tenofovir disoproxil (TDF), or to TDF/FTC/EFV, the local standard of care. For this analysis follow-up lasted to week 192 [2]. Participants had to be at least 12 years old and could not have taken ART in the past 6 months. No one had genotyping for resistance mutations when they entered the trial.
The TAF/FTC+DTG group, the TDF/FTC+DTG group, and the TDF/FTC/EFV group all had an initial median age of 32 years. About 60% across these three treatment arms were women, median weight stood at about 66 kg, and median body mass index at 24.1 kg/m2-just below the overweight threshold. Median starting CD4 counts were similar in the three study groups (average 337), and just over three quarters of participants had an initial HIV load below 100,000 copies. In the 48-week analysis, proportions of people in each study arm with a viral load below 50 copies were 84% with TAF/FTC+DTG, 85% with TDF/FTC+DTG, and 79% with TDF/FTC/EFV, results establishing the virologic noninferiority of the DTG regimens to the standard-of-care EFV combination [2].
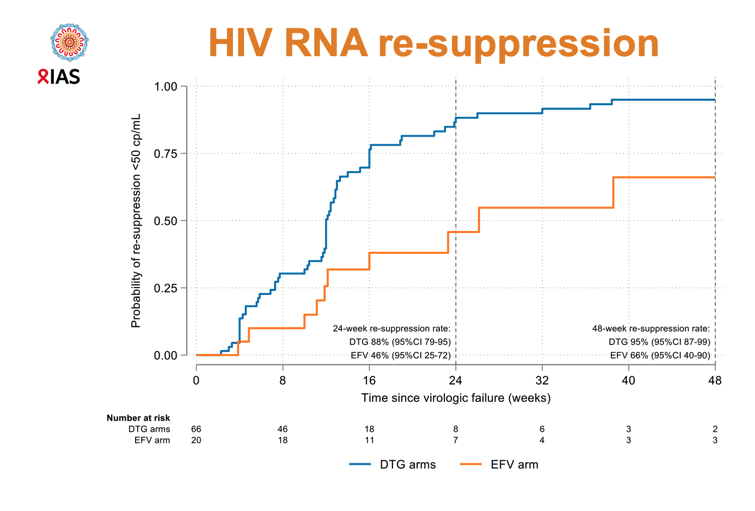
For the new viral resuppression analysis, ADVANCE researchers defined viral suppression as the first viral load below 50 copies. Virologic failure meant 1 viral load of at least 1000 copies at least 24 weeks after treatment began. Resuppression meant regaining a viral load below 50 copies after virologic failure as just defined.
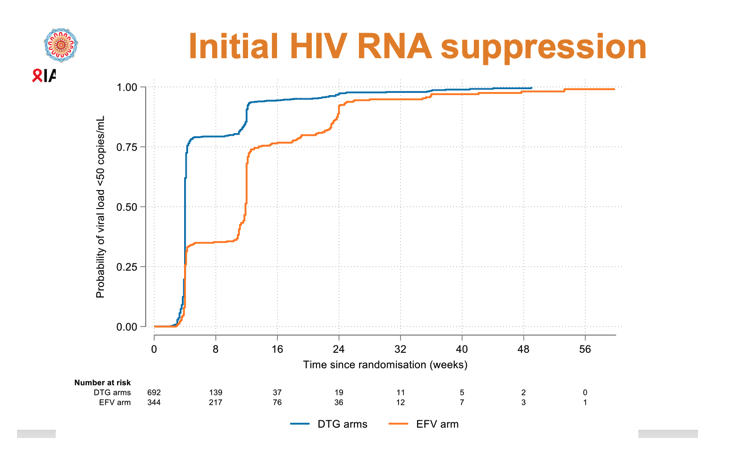
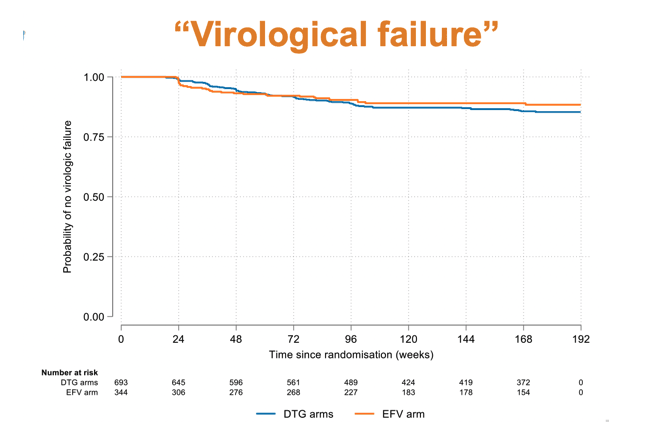
Initial viral suppression came more quickly in the combined DTG groups than in the EFV group (75% below 50 copies at about week 4 with DTG versus about week 12 with EFV). By week 24 viral suppression rates were high and equivalent in all three study arms. Through 192 weeks of follow-up, virologic failure rates were low (about 12%) and overlapping in the DTG arms and the EFV arm.
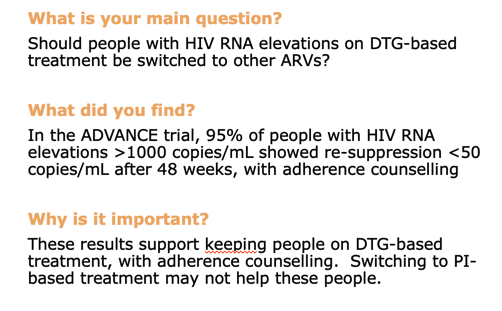
But among people with virologic failure, resuppression rates proved substantially higher in the combined DTG groups than in the EFV group 24 weeks after virologic failure (88%, 95% confidence interval [CI] 79 to 95, vs 46%, 95% CI 25 to 72) and 48 weeks after virologic failure (95%, 95% CI 87 to 99, vs 66%, 95% CI 40 to 90).
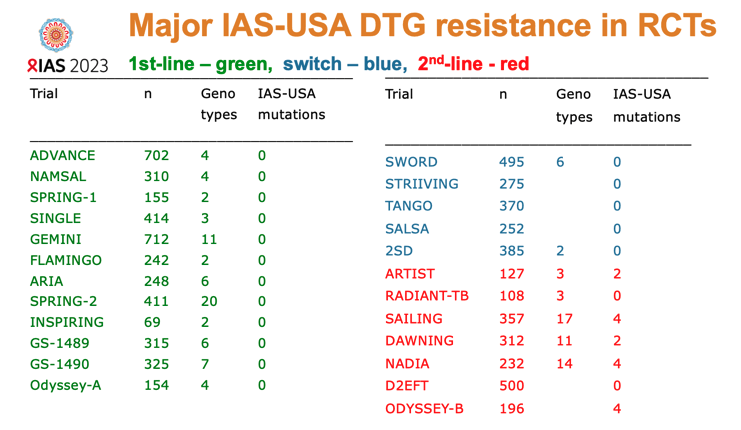
Genotyping of study participants with virologic failure (viral load above 1000 copies) found no integrase inhibitor-related resistance mutations in people taking DTG. The researchers noted, though, that “a significant percentage” of people stopped returning for study visits after virologic failure, and not everyone with a viral load above 1000 copies got genotyped. Meta-analysis of 12 trials of first-line DTG confirmed the lack of genotypic integrase inhibitor resistance after first-line failure of this integrase inhibitor. But integrase inhibitor-associated mutations did arise in 5 of 7 trials of second-line DTG.
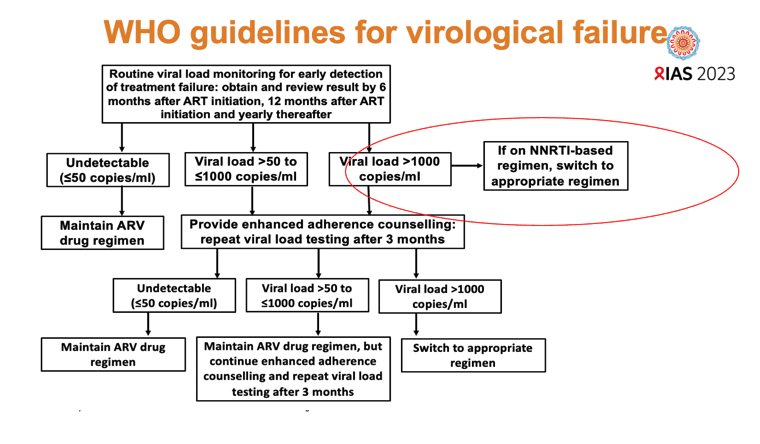
In email to NATAP, principal investigator Andrew Hill (University of Liverpool) suggested these findings raise questions about the need to switch from a first-line DTG regimen to a second-line protease inhibitor (PI) if viral load rebounds from undetectability. “It looks like adherence counselling would be enough for most people,” he proposed, “and the DTG will probably be better tolerated than PIs anyway.” The ADVANCE investigators noted that new South African antiretroviral guidelines advise switching people off DTG only if genotyping detects integrase inhibitor-related mutations.
References
1. Bosch B, Sokhela S, Akpomiemie G, et al. High rates of long-term HIV RNA re-suppression after virological failure on dolutegravir in the ADVANCE trial. IAS 2023, July 23-26, 2023. Brisbane.
2. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381:803-815. DOI 10.1056/NEJMoa1902824. https://www.nejm.org/doi/full/10.1056/NEJMoa1902824

|
| |
|
 |
 |
|
|