 |
 |
 |
| |
Associations between antiretroviral regimen and
changes in blood pressure: results from the D2EFT study
|
| |
| |
D2EFT: DOLUTEGRAVIR AND DARUNAVIR EVALUATION IN ADULTS FAILING FIRST-LINE NNRTI THERAPY CROI 2023
Bigger Blood Pressure Jump With DTG 2nd-Line Therapy Trial
IAS 2023, July 23-26, 2023. Brisbane
Mark Mascolini
In a randomized second-line therapy trial comparing three regimens, two groups taking the integrase inhibitor dolutegravir (DTG) had bigger jumps in blood pressure through 48 weeks than a group taking ritonavir-boosted darunavir (DRV/r) plus two nucleos(t)ides (NRTIs) [1]. But the biggest blood pressure gain came with second-line DTG plus DRV/r with no NRTIs.
Hypertension remains a concerning comorbidity of HIV infection in the current antiretroviral era, but little work has compared the impact of different antiretrovirals or antiretroviral classes on blood pressure. Kathy Petoumenos of Sydney’s Kirby Institute and international colleagues who worked on the D2EFT trial [2] used data from the three arms of that study to compare 48-week blood pressure changes from one regimen to the next.
D2EFT randomized people taking a failing nonnucleoside regimen to (1) a standard of care (SOC) arm including DRV/r plus two NRTIs, (2) DTG plus DRV/r, or (3) DTG with tenofovir disoproxil and lamivudine or emtricitabine (DTG + NRTIs). The blood pressure analysis included D2EFT participants with systolic and diastolic blood pressure and body mass index (BMI) recorded at the baseline study visit and at week 48. For the primary analysis, no one could have baseline hypertension, defined as systolic blood pressure at or above 140 mm Hg, diastolic blood pressure at or above 90 mm Hg, or reported hypertension.
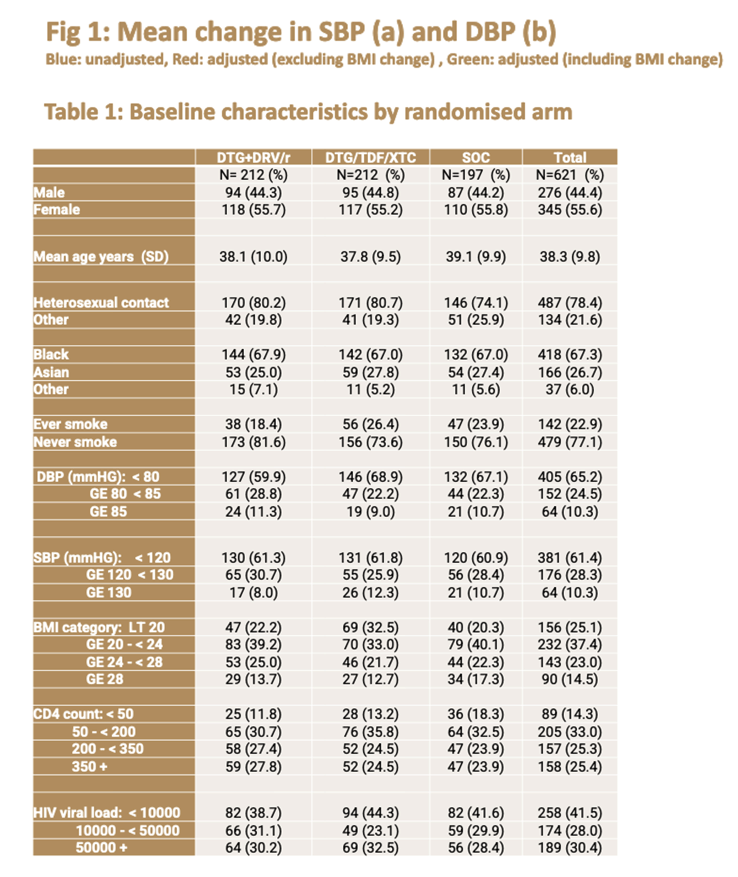
This analysis included 621 of the original 826 D2EFT participants, 197 in the SOC arm, 212 in the DTG + DRV/r arm, and 212 in the DTG + NRTI arm. About 44% in all three groups were men, 67% black, about 27% Asian, and age averaged about 38 years.
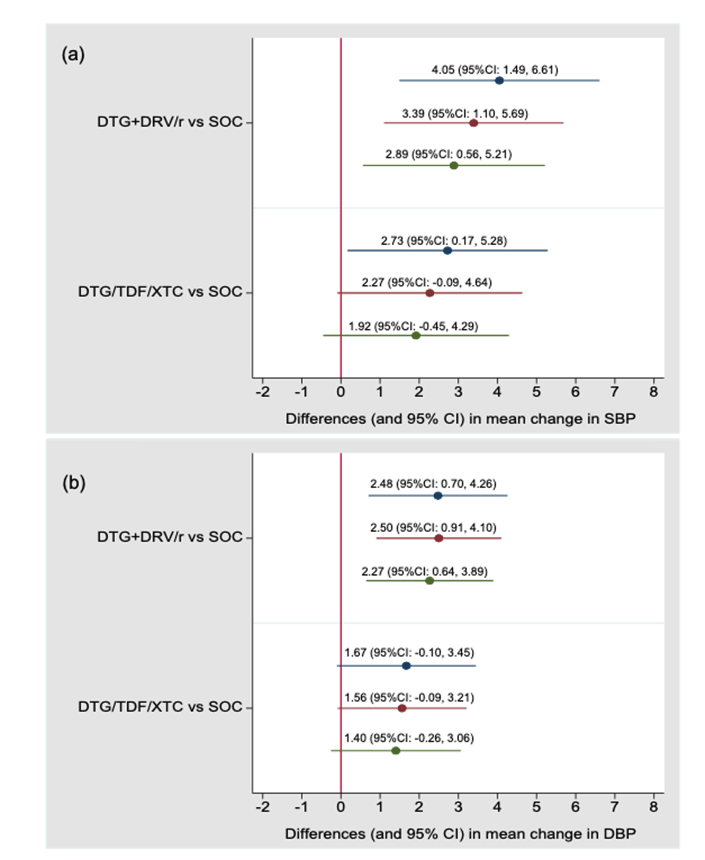
After 48 weeks systolic blood pressure gains with SOC, DTG + DRV/r, and DTG+NRTI averaged 2.68, 6.73, and 5.41 mm Hg, while diastolic gains averaged 1.36, 3.83, and 3.03 mm Hg.
To compare systolic or diastolic blood pressure changes between study arms, the researchers used multivariate linear regression analyses adjusted for BMI change over time and a host of baseline variables: age, sex, race, smoking status, study stage, systolic blood pressure (for systolic change), and diastolic blood pressure (for diastolic change).
People randomized to DTG + DRV/r had significantly greater average systolic and diastolic blood pressure gains than those randomized to SOC after adjustment for baseline variables and, in addition, for BMI change.
Change in systolic blood pressure DTG + DRV/r vs SOC
Unadjusted: difference 4.05, 95% confidence interval (CI) 1.49 to 6.61
Adjusted for baseline variables: difference 3.39, 95% CI 1.10 to 5.69
Adjusted for baseline variables and BMI change: difference 2.89, 95% CI 0.56 to 5.21
Change in diastolic blood pressure DTG + DRV/r vs SOC
Unadjusted: difference 2.48, 95% CI 0.70 to 4.26
Adjusted for baseline variables: difference 2.50, 95% CI 0.91 to 4.10
Adjusted for baseline variables and BMI change: difference 2.27, 95% CI 0.64 to 3.89
Average changes in systolic and diastolic blood pressure were greater with DTG + NRTIs than with SOC, but these differences did not reach statistical significance. Neither sex nor ethnicity had statistically significant interactions with study arm. Including people who had hypertension when the study began did not change results.
D2EFT researchers stressed that the pathophysiology of blood pressure changes with different antiretroviral classes needs further scrutiny. They encouraged hypertension screening for people with HIV, as well as lifestyle and behavioral strategies to prevent weight gain.
References
1. Associations between antiretroviral regimen and changes in blood pressure: results from the D2EFT study. IAS 2023, July 23-26, 2023. Brisbane. Abstract
2. Papot E, Jacoby S, Arlinda D, et al. Adaption of an ongoing clinical trial to quickly respond to gaps in changing international recommendations: the experience of D2EFT. HIV Res Clin Pract. 2022;23:37-46. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10029359/

|
| |
|
 |
 |
|
|