 |
 |
 |
| |
Suppressed switch to bictegravir/emtricitabine/tenofovir
alafenamide vs dolutegravir/lamivudine
DTG/3TC Stopped More Than B/F/TAF After Switch From Suppressive Regimen
|
| |
| |
IDWeek 2023, October 11-15, 2023, Boston
Mark Mascolini
People who switched from a virologically suppressive regimen to dolutegravir/lamivudine (DTG/3TC) stopped that combination sooner than people who switched to bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) in OPERA, a large US database of people with HIV [1]. But antiviral effectiveness or safety and tolerability did not appear to explain discontinuations of either regimen.
B/F/TAF has emerged as the most-prescribed antiretroviral regimen in the United States, while DTG/3TC ranks as the favorite two-drug combo. Trials that randomized people to stay with a current suppressive regimen or switch to B/F/TAF or DTG/3TC found both of these popular combinations effective, safe, and tolerable.
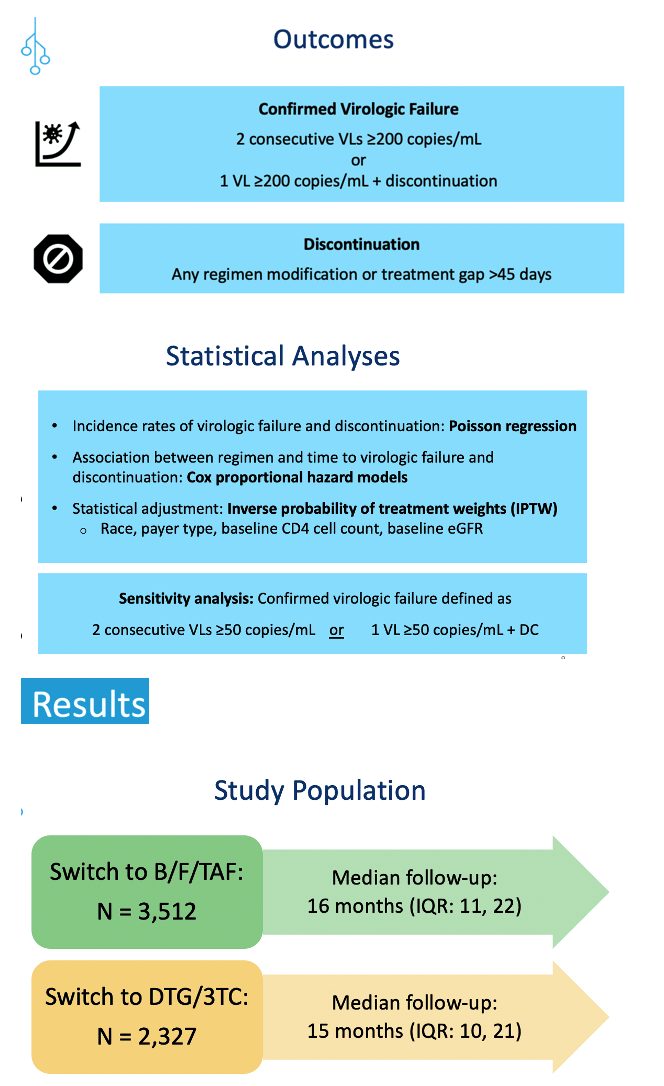
For this analysis researchers sifted electronic medical records in OPERA to find adults who switched to B/F/TAF or DTG/3TC between August 2020 and June 2022 from a regimen keeping their viral load below 200 copies. Follow-up continued until December 2022 or a person stopped the new regimen, stopped making OPERA visits, or died. Researchers defined confirmed virologic failure as two consecutive viral loads at or above 200 copies or one viral load at that level and stopping the switch-to combination. Discontinuation meant any change to a regimen or a treatment gap more than 45 days.
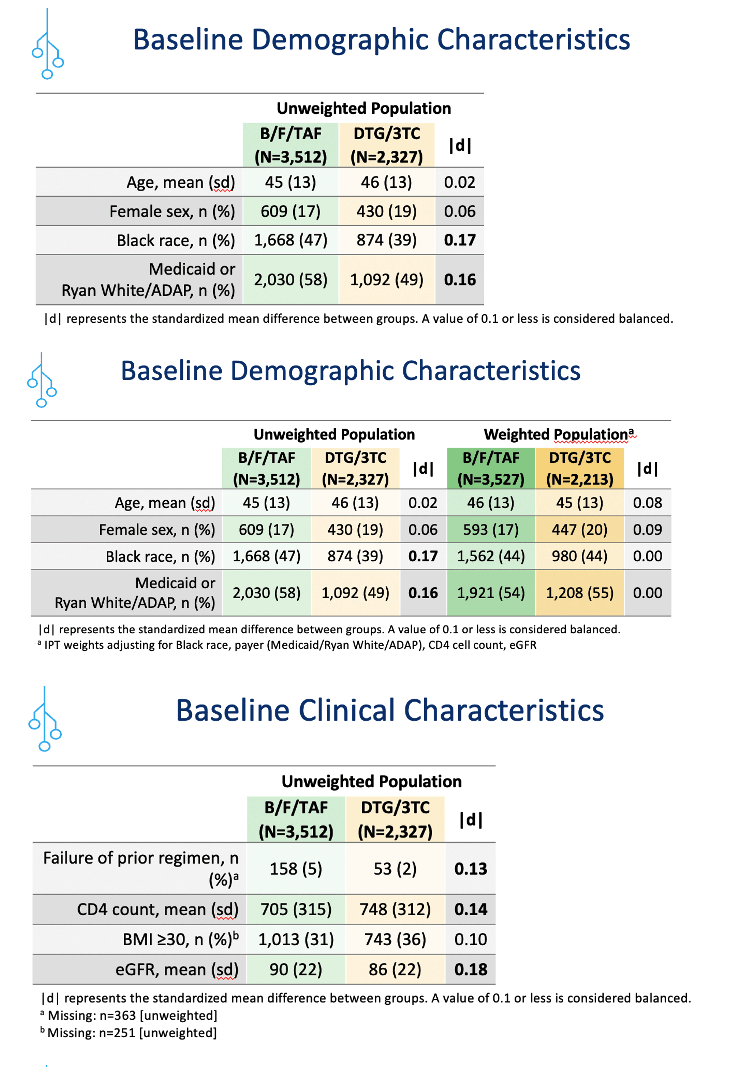
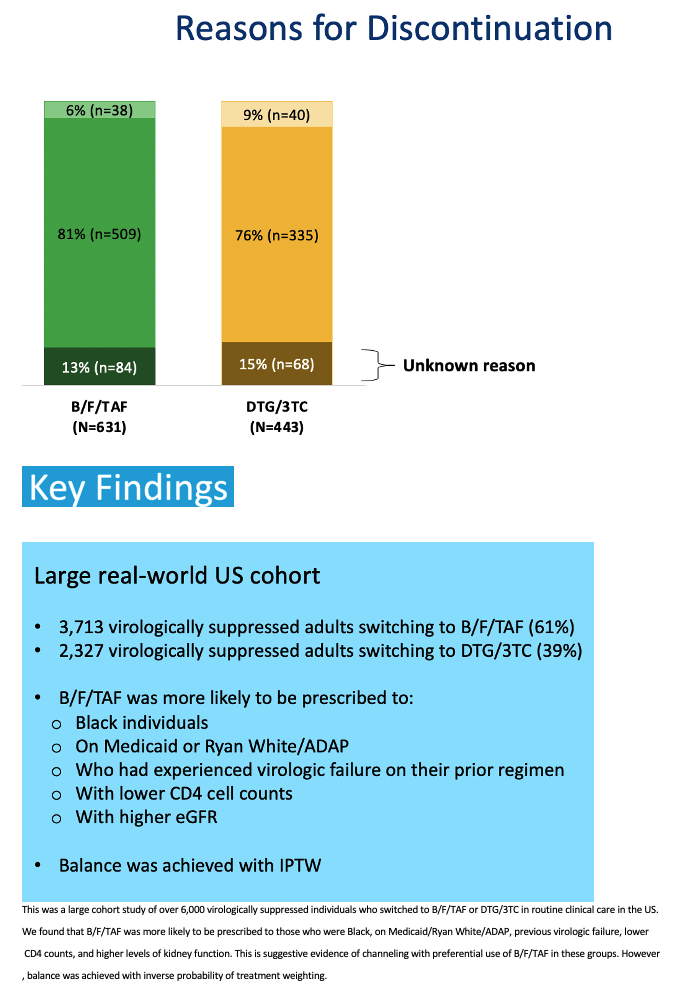
The study involved 3713 people who switched to B/F/TAF and had a median follow-up of 16 months and 2327 people who switched to DTG/3TC and had a median follow-up of 15 months. To create treatment populations balanced in age, proportions of women and blacks, and use of Medicaid or Ryan White/AIDS Drug Assistance Program, the researchers used inverse probability weighting adjusting for black race, payer, CD4 count, and estimated glomerular filtration rate (eGFR), a measure of kidney function. The weighted B/F/TAF and DTG/3TC populations averaged 46 and 45 years in age and included 17% and 20% women, 44% and 44% blacks, and 54% and 55% using Medicaid or Ryan White to pay for care.
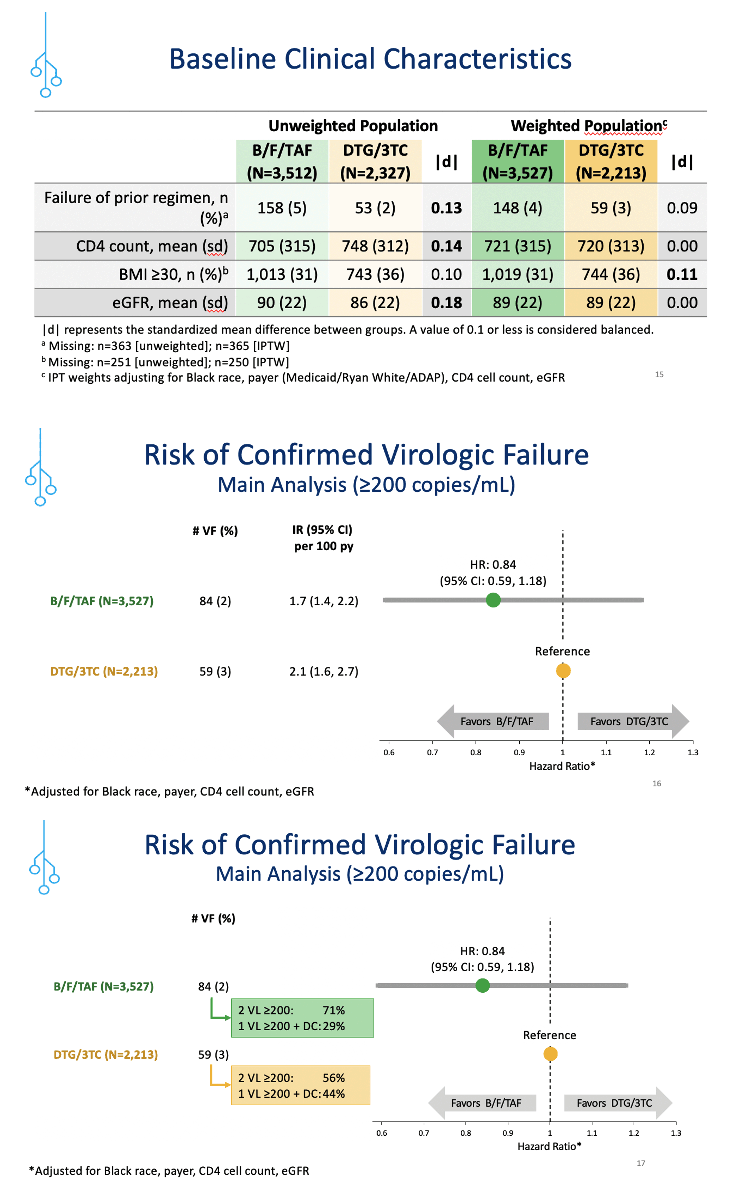
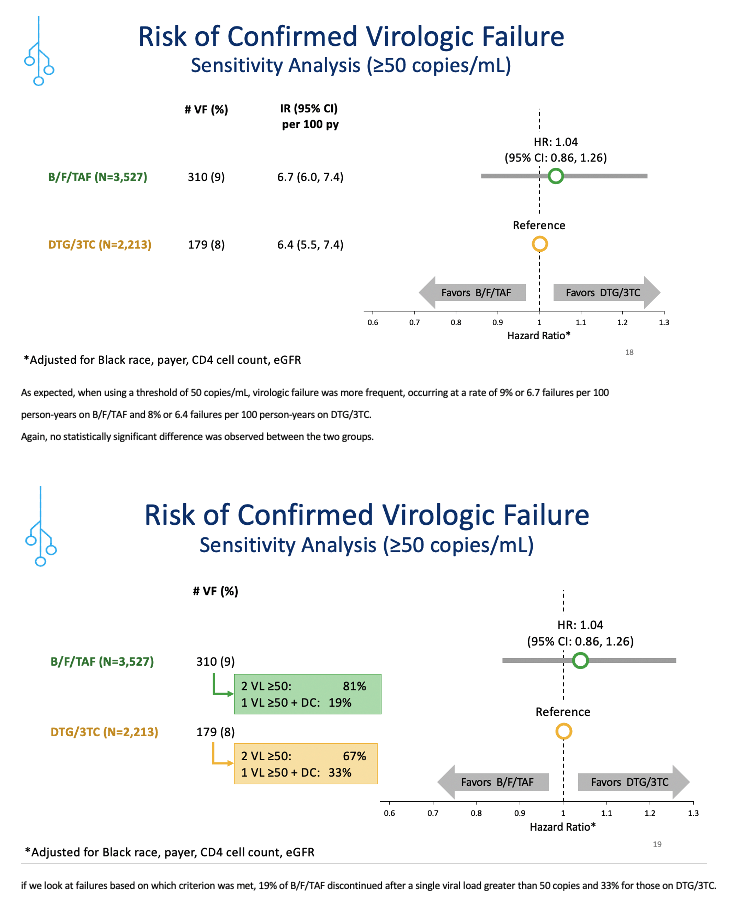
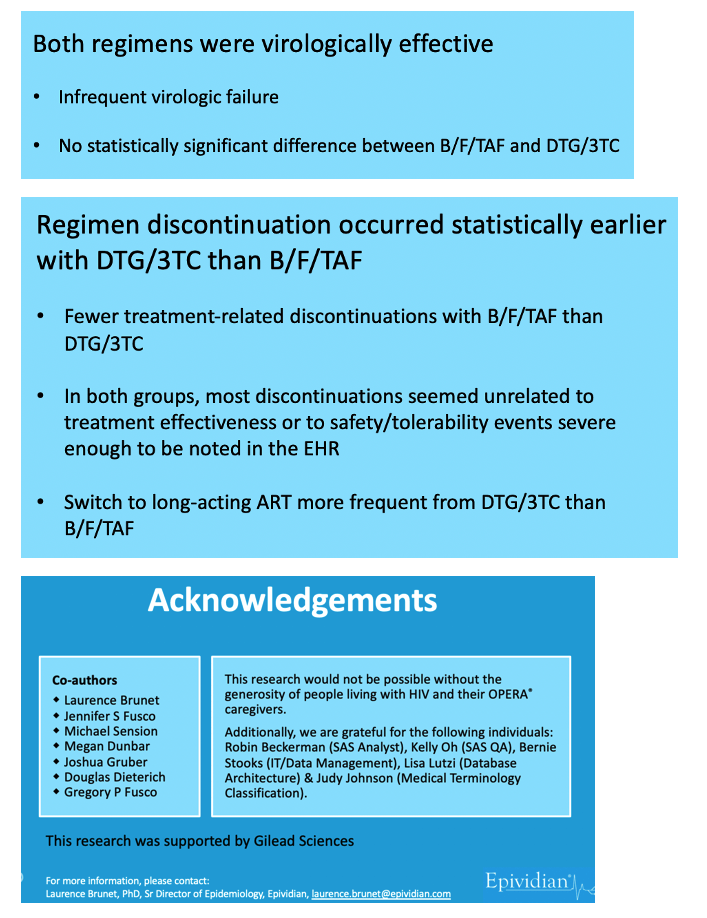
Confirmed virologic failure rates measured 2% in the B/F/TAF group and 3% in the DTG/3TC group, resulting in failure incidence of 1.7 per 100 person-years with B/F/TAF and 2.1 per 100 person-years with DTG/3TC. The resulting hazard ratio (HR) favored B/F/TAF but not significantly (HR 0.84, 95% confidence interval [CI] 0.59 to 1.18). A sensitivity analysis using a virologic failure cutoff of 50 copies (rather than 200 in the primary analysis) determined that the hazard ratio shifted to favor DTG/3TC, but again the difference lacked statistical significance (HR 1.04, 95% CI 0.86 to 1.26).
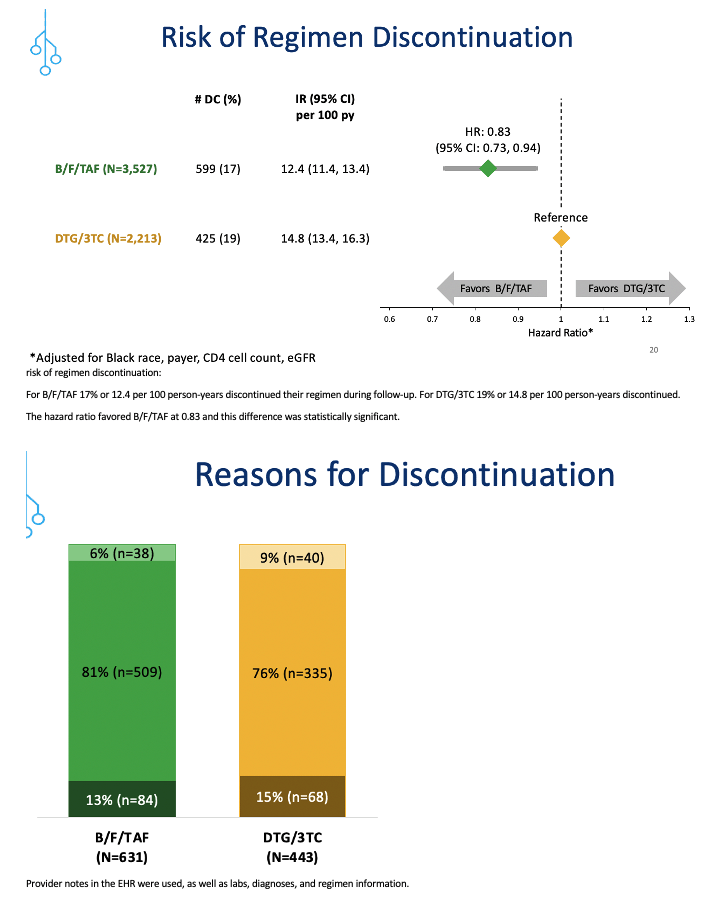
But the regimen discontinuation rate did significantly favor B/F/TAF, standing at 17% or 12.4 per 100 person-years, compared with 19% or 14.8 per 100 person-years with DTG/3TC. Those numbers translated into a 17% lower risk of regimen discontinuation with B/F/TAF (HR 0.83, 95% CI 0.73 to 0.94).
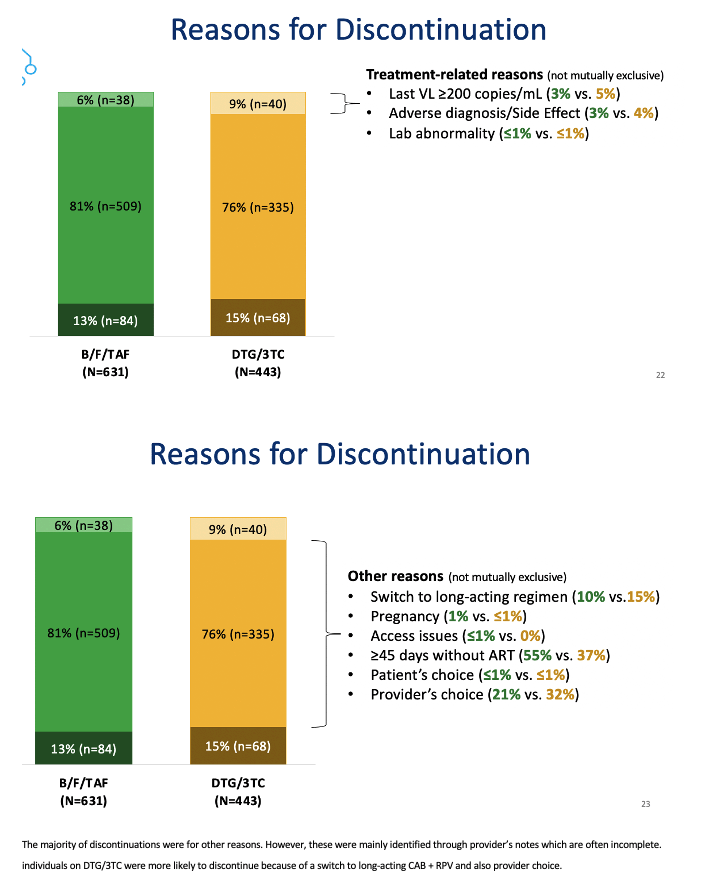
The researchers used lab results, diagnoses, regimen information, and provider notes to assign reasons for stopping a regimen. Only 6% in the B/F/TAF group and 9% in the DTG/3TC group stopped therapy for treatment-related reasons (latest viral load above 200 copies, an adverse diagnosis or side effects, or lab abnormalities). The biggest chunk of discontinuations (81% with B/F/TAF and 76% with DTG/3TC) involved "other reasons," such as switching to a long-acting regimen, pregnancy, and access problems. The remaining small share of discontinuations had unknown reasons.
Clinicians were more likely to prescribe B/F/TAF than DTG/3TC for black individuals, people using Medicaid or Ryan White/AIDS Drug Assistance Program, those who had virologic failure with their previous combination, and those with lower CD4 counts and higher eGFR.
The researchers stressed that both study regimens were virologically effective and rarely had to be stopped for virologic failure, so reasons for the higher discontinuation rate with DTG/3TC remain obscure. The investigators observed that a higher proportion of people switched from DTG/3TC than B/F/TAF to long-acting cabotegravir plus rilpivirine. That had to contribute to the higher discontinuation rate with DTG/3TC, but then one is left with the question why a higher proportion taking DTG/3TC than B/F/TAF opted to move on to the long-acting injectables.
Reference
1. Pierone G Jr, Brunet L, Fusco JS, et al. Suppressed switch to bictegravir/emtricitabine/tenofovir alafenamide vs dolutegravir/lamivudine. IDWeek 2023, October 11-15, 2023, Boston.

|
| |
|
 |
 |
|
|