 |
 |
 |
| |
HBV Screening and Vaccination Dropping in PrEP Users; Vaccinations Adherence
|
| |
| |
IDWeek 2023, October 11-15, 2023, Boston
Mark Mascolini
Adherence to guidelines for hepatitis B virus (HBV) screening and vaccination fell in recent years, according to analysis of 290 people prescribed PrEP to avoid HIV infection [1]. This single-center study at the University of Illinois at Chicago found that vaccination against human papillomavirus (HPV) improved in PrEP users in 2018-2022 compared with 2014-2018.
The US Public Health Service updated PrEP guidelines in 2021 [2]. In early PrEP years, a 2011 survey of 189 HIV clinicians found that more than three quarters knew about CDC guidance on PrEP, but only 61% of respondents screened PrEP users for HBV [3]. A 2017 analysis of a commercial insurance database found only 38% adherence to recommended HBV screening before starting PrEP [4].
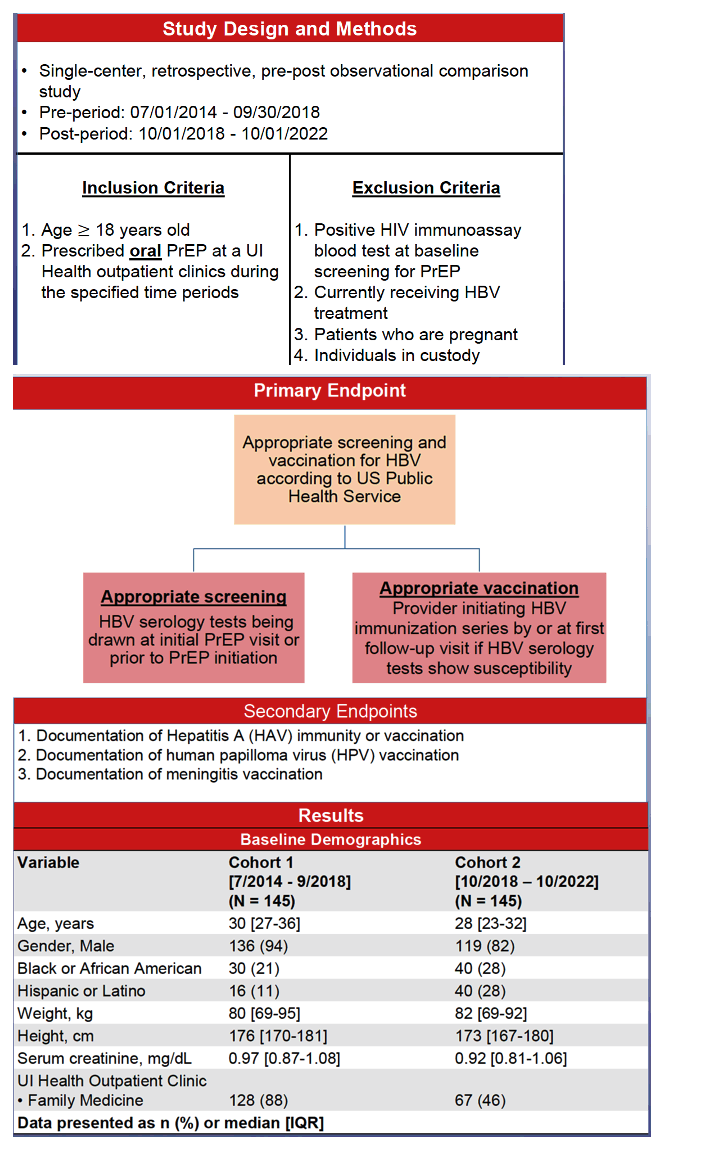
This retrospective observational study compared adherence to US Public Health Service guidelines on screening and vaccination for HBV in adults prescribed oral PrEP at University of Illinois outpatient clinics in two periods: July 1, 2014 to September 30, 2018 (early cohort) and October 1, 2018 to October 1, 2022 (later cohort). The researchers also compared the two groups for guideline adherence on evaluating hepatitis A virus (HAV) immunity or vaccination, HPV vaccination, and meningitis vaccination.
The early and later cohorts each included 145 people with median ages of 30 and 28 years and weights of 80 and 82 kg. Majorities in the early cohort (94%) and the late cohort (82%) were men, 21% and 28% black, and 11% and 28% Hispanic.
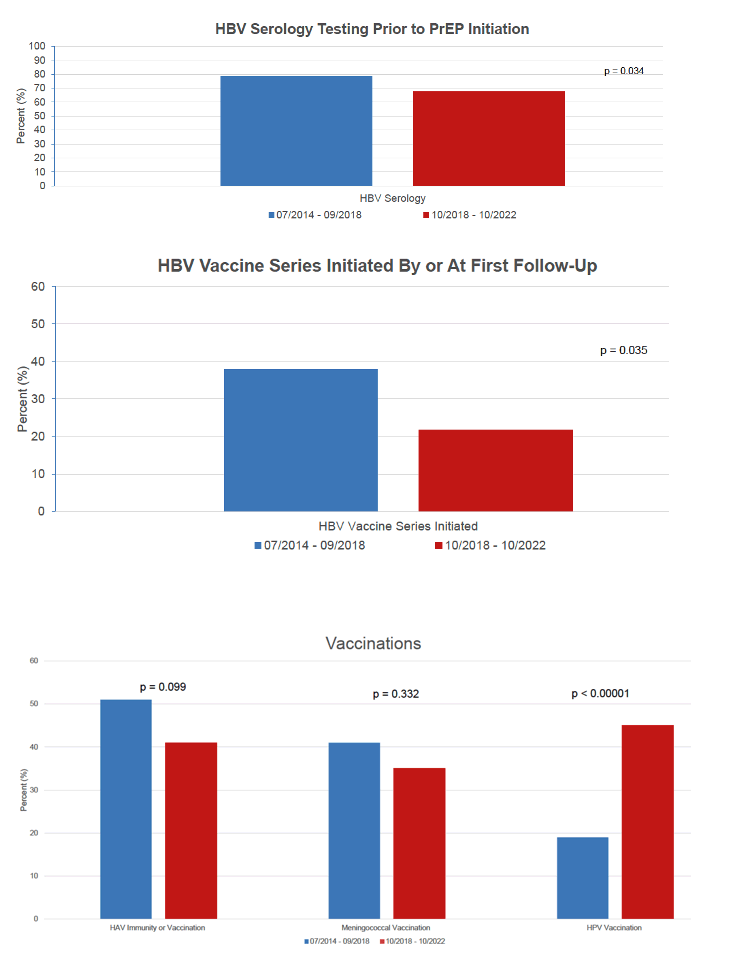
A significantly higher proportion of clinicians caring for the early versus the later cohort conducted recommended HBV serology testing before starting PrEP (about 80% versus 70%, P = 0.034). About twice as many PrEP users in the early cohort than the later cohort had a recommended HBV vaccine series started by or at first follow-up (about 40% versus 20%, P = 0.035).
A higher proportion in the early cohort than the later cohort also got evaluated for HAV immunity or had HAV vaccination, but this difference did not reach statistical significance (about 50% versus 40%, P = 0.099). And a slightly higher proportion in the early group than in the later group got recommended meningococcal vaccination (about 40% versus 35%, P = 0.332). The only guideline adherence outcome in which the later cohort did better than the early cohort was HPV vaccination (about 20% early versus 45% later, P < 0.00001), possibly a signal of growing clinical awareness of the value of HPV vaccination in preventing cervical, anal, vaginal, and vulvar cancer.
References
1. Awad R, Michienzi S, Badowski M, et al. Guideline adherence to hepatitis B virus screening and vaccination in patients prescribed HIV PrEP. IDWeek 2023, October 11-15, 2023, Boston.
2. Centers for Disease Control and Prevention: US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States-2021 update: a clinical practice guideline. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
3. Tellalian D, Maznavi K, Bredeek UF, et al. Pre-exposure prophylaxis (PrEP) for HIV infection: results of a survey of HIV healthcare providers evaluating their knowledge, attitudes, and prescribing practices. AIDS Patient Care STDS. 2013;27:553-559. https://www.liebertpub.com/doi/10.1089/apc.2013.0173
4. Huang Y-LA, Tao G, Samandari T, Hoover KW. Laboratory testing of a cohort of commercially insured users of HIV preexposure prophylaxis in the United States, 2011-2015. J Infect Dis. 2017;217:617-621. https://pubmed.ncbi.nlm.nih.gov/29145597


|
| |
|
 |
 |
|
|