 |
 |
 |
| |
Strong Oral PrEP Uptake and Persistence in
3000 African Girls and Young Women in INSIGHT Cohort
|
| |
| |
CROI 2024 (Conference on Retroviruses and Opportunistic Infections), March 3-6, 2024, Denver
Mark Mascolini
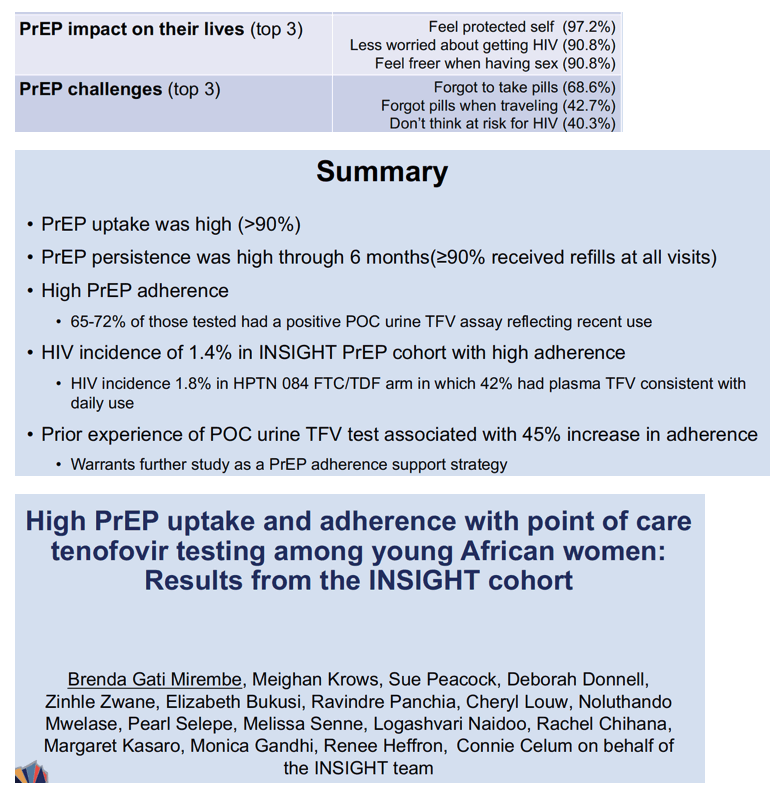
Among 3087 adolescent girls and young women (AGYW) offered oral TDF/FTC preexposure prophylaxis (PrEP) in the African INSIGHT cohort, 93% started PrEP, and 96%, 95%, and 90% got refills at months 1, 3, and 6 [1]. The study, which purposely recruited sexually active AGYW interested in PrEP, used a novel urine tenofovir (TNF) assay to check TDF/FTC adherence objectively. INSIGHT researchers suggested the fast test can further support adherence.
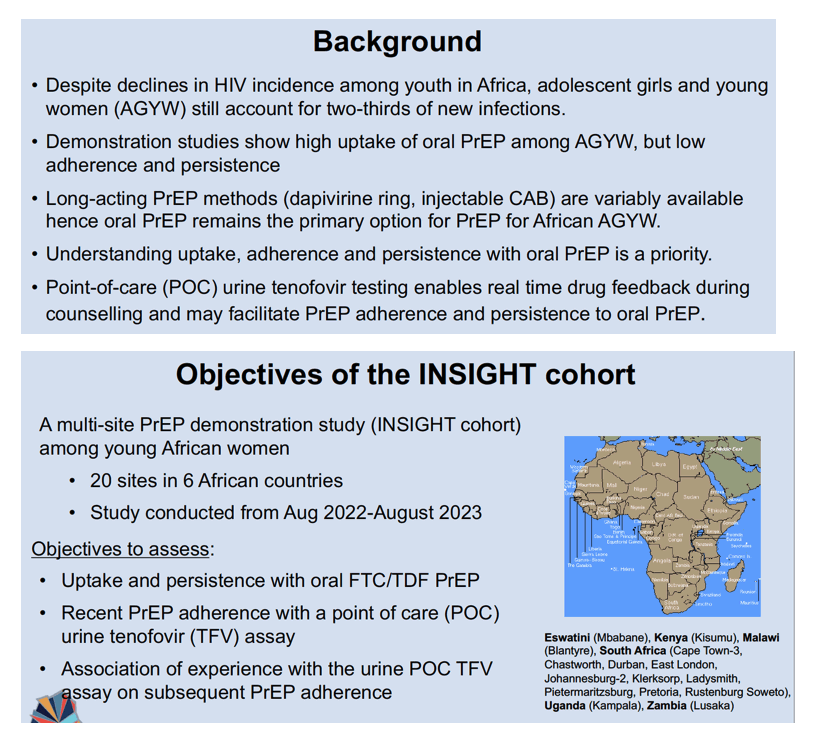
Brenda Mirembe (Makerere University, Kampala) and INSIGHT colleagues noted that AGYW account for two thirds of new HIV infections in sub-Saharan Africa. Many AGYW are eager to try PrEP, the researchers said, but often fail to keep taking the daily pills. Long-acting PrEP strategies like the dapivirine ring and injected cabotegravir remain beyond the reach of most in this population, so oral PrEP is their only medication-based option to ward off HIV.
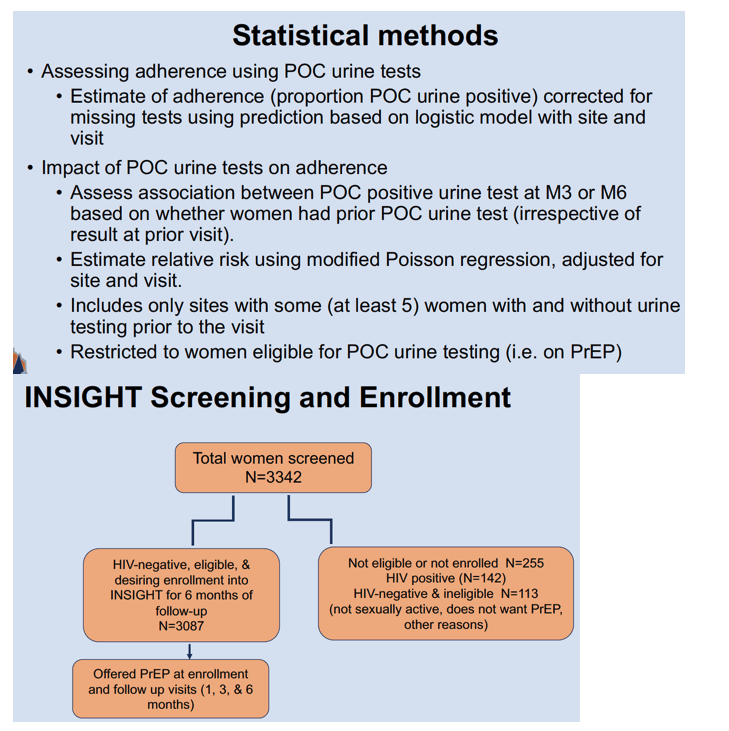
INSIGHT-a PrEP demonstration project-recruited AGYW from 20 sites in 6 African countries. Conducted from August 2022 to August 2023, the study assessed uptake and persistence of oral TDF/FTC PrEP. Cohort members self-reported TDF/FTC adherence and often agreed to a point-of-care urine TNF assay that allows AGYW to get real-time drug-taking feedback during counseling. The assay takes 60 seconds to detect TNF levels above 1500 ng/mL. Results come back positive for 98% of people who took TDF in the past 4 days and reflect liquid chromatography-mass spectrometry (the standard drug-level test) at 6 months.
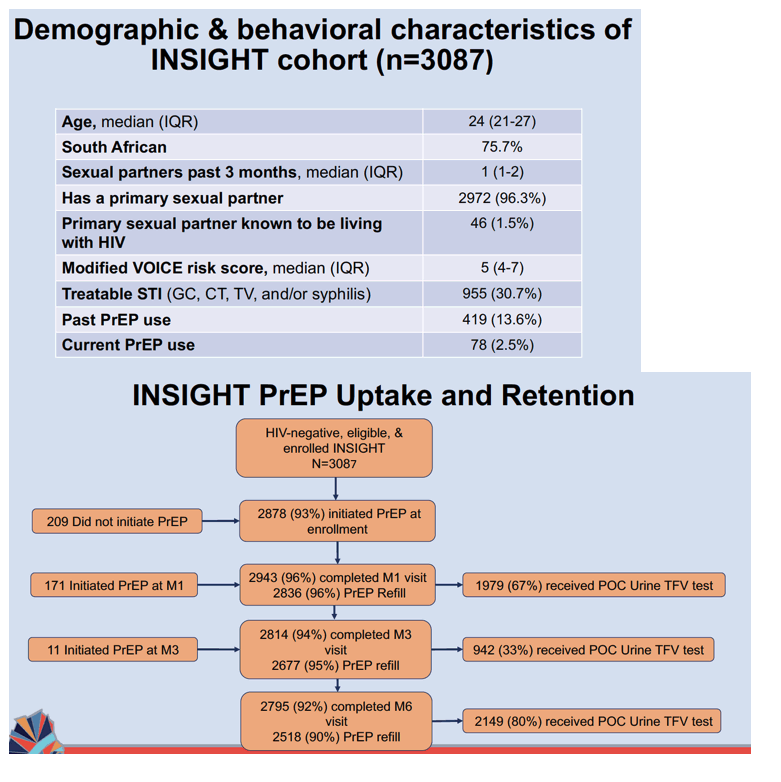
These 3087 participants had a median age of 24 (interquartile range 21 to 27), and 75.7% lived in South Africa. While 96.3% had a primary sex partner, 30.7% had a treatable sexually transmitted infection, 13.6% used PrEP earlier, and 2.8% used PrEP when enrolling in this study.
Of these 3087 AGYW, 2878 (93%) began PrEP, 2943 (95%) completed the month-1 visit, and
2836 (96% of 2943) got a PrEP refill after 1 month. Of the 2814 AGYW who completed the month-3 visit, 2677 (95%) got a PrEP refill. Among 2795 AGYW who completed the month-6 visit, 2518 (90%) got a PrEP refill. At months 1, 3, and 6, 1979 (67% of 2943) participants, 942 (33% of 2814), and 2149 (77% of 2795) got the point-of-care TNF urine assay.
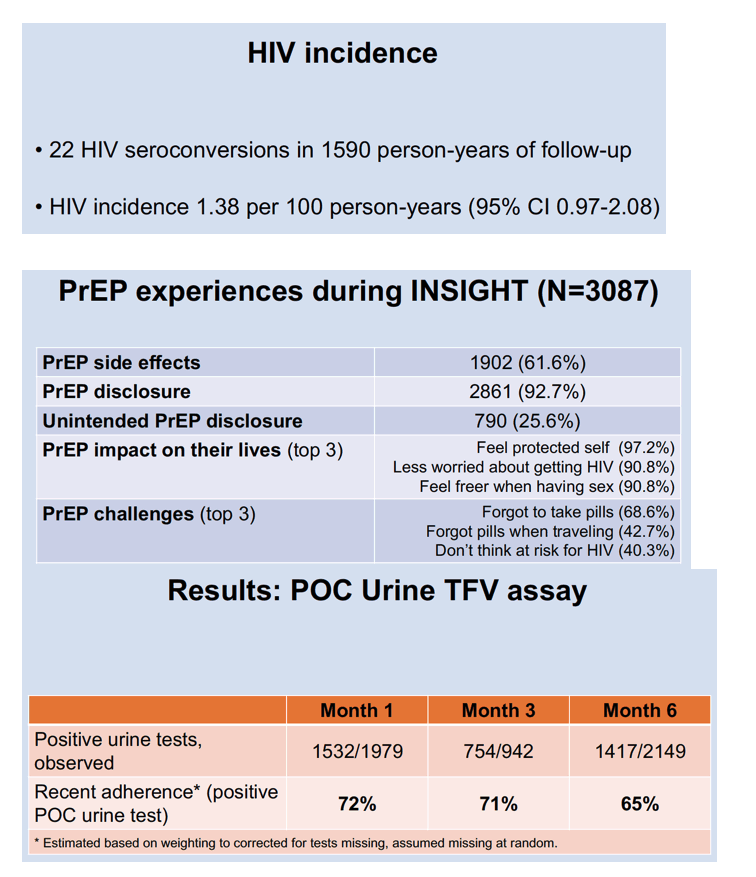
Twenty-two study participants acquired HIV infection during 1590 person-years of follow-up for an HIV incidence of 1.38 per 100 person-years (95% confidence interval [CI] 0.97 to 2.08).
Among 3087 AGYW who enrolled in INSIGHT, 1902 (61.6%) reported PrEP side effects, 2861 (92.7%) disclosed that they were using PrEP, and 790 (25.6%) unintentionally disclosed PrEP use.
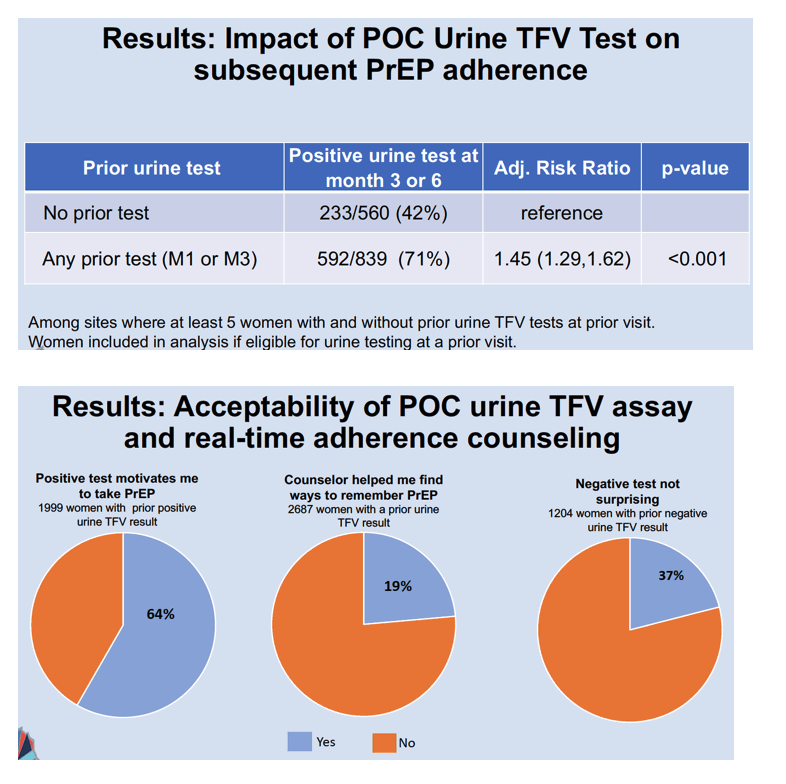
Urine assays proved positive for TFV in 1532 of 1979 AGYW at month 1, in 754 of 942 at month 3, and in 1417 of 2149 at month 6. After weighting to correct for missing tests, INSIGHT statisticians figured positive urine test rates indicating TDF/FTC adherence of 72% at month 1, 71% at month 3, and 66% at month 6. Among AGYW with no prior urine test, 233 of 560 (42%) had a positive test at month 3 or 6. Among 839 AGYW with a urine test in month 1 or 3, 592 (71%) had a positive test at month 3 or 6. Comparing the 42% and 71% positive rates, the researchers calculated that AGYW with a positive urine test at month 1 or 3 had a 45% greater chance of subsequent PrEP adherence (adjusted risk ratio 1.45, 95% CI 1.29 to 1.62, P < 0.001).
Almost two thirds of participants (64%) said a positive urine test motivated them to take PrEP, 19% credited adherence counselors with helping them find ways to remember PrEP, and 37% said a negative urine test did not surprise them.
INSIGHT investigators listed study limitations as lack of randomization for urine TNF testing and the possibility that taking a TDF/FTC dose just before a study visit could have contributed to positive urine TNF results. Researchers estimate such “white coat dosing” at 5% to 30% in previous PrEP studies. The investigators stressed that adherence and persistence must be tested beyond the 6 months of this study.
With these limitations in mind, the INSIGHT team concluded that both PrEP uptake and self-reported 6-month persistence were high-around 90%-in this cohort. Because the study linked a positive point-of-care urine TFV test to a 45% greater likelihood of adherence, the researchers suggested urine testing deserves further study as a simple and fast PrEP adherence measure. INSIGHT clearly shows that motivated adolescent girls and young women can start and continue TDF/FTC at high rates in the context of a well-conducted study.
Reference
1. Mirembe BG, Krows M, Zwane Z, et al. High PrEP uptake and adherence measured objectively among young African women in the INSIGHT cohort. CROI 2024 (Conference on Retroviruses and Opportunistic Infections), March 3-6, 2024, Denver. Abstract 167.


|
| |
|
 |
 |
|
|