 |
 |
 |
| |
Summary from CROI 2024 for HIV and liver disease
|
| |
| |
What is new in viral hepatitis coinfection? Is HBV cure on its way? Can HCV elimination be achieved?
Are there new treatment options for metabolic dysfunction-associated liver disease (MASLD)?
New insights from CROI 2024.
Jurgen K. Rockstroh M.D., Professor of Medicine
University of Bonn, Germany
Correspondence:
Prof. Dr. Jurgen K. Rockstroh
Department of Medicine I
University Hospital Bonn
Venusberg-Campus 1
53127 Bonn
Germany
Introduction
The 31st CROI conference was back in Denver (previous last CROI in Denver was 2006) after 18 years and took place as a mainly f-2-f meeting with an impressive 3635 delegates and an additional 438 virtual attendees. After a period of decreased abstract submission due to the impact of the Covid-19 pandemic, science and research activities have now substantially resumed and an amazing 1682 abstracts were submitted to this year CROI, with 966 being accepted. HIV and liver disease remained an important topic this year but had to compete with many other areas of interest. As last year, the oral abstract session for hepatitis research was integrated into the session on tuberculosis and hepatitis but the number of presentations from the HIV and liver disease area increased this year. Abstracts presented in the oral session covered risk factors and new treatment options for MASLD, preclinical pharmacokinetic assessment of a HCV long-acting injectable formulation, HCV prevention efforts and incidence among people who inject drugs and insights into intrahepatic HDV activity (1-5). In addition, there was a late-breaker abstract on seroprotection rates following vaccination with a new HBV vaccine in PLWH in the special late breaker session on Wednesday (6), an interactive Symposium on new frontiers in hepatitis B (7) and a case-based workshop on the liver and its complications on Sunday preceding the opening of the conference (8). Moreover, there were numerous interesting poster presentations, which focused on various aspects of liver disease in HIV and were part of several poster sessions throughout the conference (9-26). (since CROI, resmetirom has received accelerated FDA approval for NASH, NAFLD, but it has not been studied yet in PWH. Jules)
The following summary aims at highlighting some of the exciting new data reported at CROI 2024 and to initiate a discussion about the impact of these new data on guidelines and clinical management of liver disease in HIV-coinfected individuals.
Hepatitis B Vaccination
HepB-CpG vaccines such as HEPLISAV-B combine 20 mcg of recombinant HBsAg with CpG 1018 adjuvant. HepB-CpG's novel adjuvant binds to TLR9, a toll-like receptor agonist that provokes innate immune responses. The FDA approved 2 doses of this vaccine for people 18 and older. Within a first study from the ATCG this new vaccine was studied in vaccine-naïve PLWH with 3 doses of the HepB-CpG vaccine (27) taking into account the historically poorer HBV vaccination responses in PLWH. Most impressively, 3 doses of the HepB-CpG vaccine provided 100% seroprotection in people with HIV and no previous hepatitis B vaccination (6). Limited data however exist so far for non-responders to conventional vaccines. ACTG A5379 is an ongoing, open-label study to evaluate immunogenicity of HepB-CpG in PWH. Prior vaccine non-responders were on ART with CD4 ≥100 cells/mm3 and HIV-1 RNA <1000 copies/mL without past or present serologic evidence of HBV or HBV vaccine response. Participants were randomized 1:1:1 to: 2 doses of HepB-CpG intramuscularly (IM) (20 mcg recombinant HBsAg, 3000 mcg CpG 1018® adjuvant) at weeks 0 and 4 (2-CpG); 3 doses of HepB-CpG IM at weeks 0, 4, 24 (3-CpG); or 3 doses of HepB-alum IM (20 mcg recombinant HBsAg) at weeks 0, 4, 24 (3-alum). Primary SPR was defined at Wk12 for 2-CpG and Wk28 for 3-CpG and 3-alum. Noninferiority (NI) of 2-CpG vs 3-alum was assessed with a 10% margin and superiority of 3-CpG vs 3-alum. Predefined primary analysis set excluded missed sample collection. Safety was also assessed.
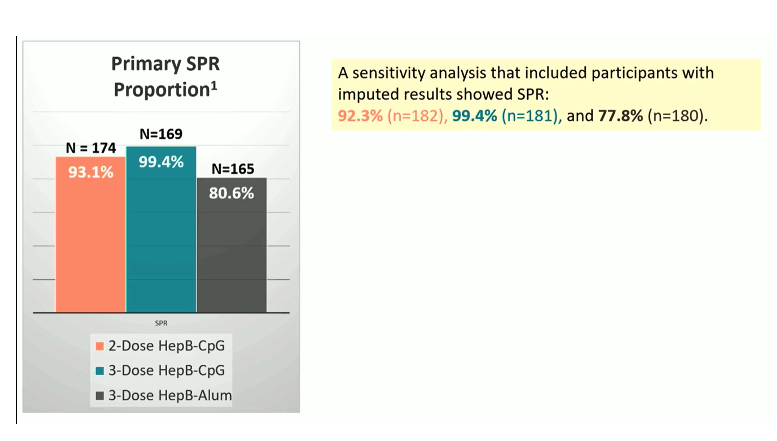
Results: Of the 561 eligible participants enrolled at 41 sites from 10 countries: 64% were male, 42% Black, 35% White, 17% Asian, 22% Hispanic. Median age was 46 years (range 18-70), 56% enrolled in the US, 21% Africa, 17% Asia, 6% S. America. Median CD4 was 638 cells/mm3, 94% had HIV-1 RNA <40 copies/ mL, 29% BMI >30, and 13% diabetes. 96% completed all prescribed doses. The seroprotection response rates are shown in figure 1. Both 2 and 3 doses of HepB-CpG achieved superior SPR compared to 3 doses of HepB-alum. Three doses of HepB-CpG achieved a higher proportion with titers >1000 mIU/ml compared to two doses, and to 3 doses of HepB-alum. No unexpected safety issues or deaths were recorded.
Figure 1: Primary results: SPR
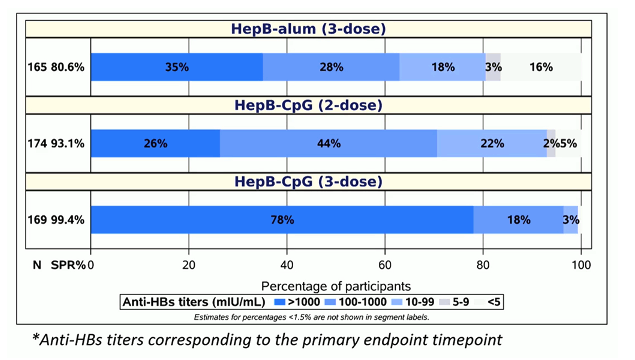
The distribution of anti-HBs- titers for all study arms is shown in figure 2. The great titers after 3 vaccinations suggests that maybe in PLWH 3 rather than 2 vaccination doses will be recommended in the setting of HIV coinfection to ensure the best seroprotection rates.
Figure 2: Distribution of Anti-HBs titers*
Conclusion: In this study of PWH with prior vaccine non-response, both 2 and 3 doses of HepB-CpG achieved superior SPR compared to 3 doses of HepB-alum. No unexpected safety issues were observed. Unfortunately, cost and reimbursement policies by health insurances currently limit the broader implementation of this new vaccine which is needed at least for non-responders to conventional HBV vaccine.
In the time period where mostly recombinant HBsAg based vaccines were used for HBV vaccination double dose (40Mg recombinant HBsAg) of standard HBV vaccine at 3 or even 4 timepoints was used as a strategy to achieve HBV seroprotection in non-responders to standard HBV vaccination. A recent trial from Taiwan demonstrated that 354 PWH who had achieved virologic and immunologic responses with ART and were randomized to receive three double dose (40-μg) HBV vaccine (Engerix) achieved a higher seroresponse rate (anti-HBs antibody titer ≥10 mIU/ml) (95.8% vs 91.5%, p=0.111) and high-titer (≥100 mIU/ml) seroresponse rate (91.7% vs 84.3%, p=0.05) than PWH who received three standard-dose (20-μg) HBV vaccine at week 28 (9). At this year CROI the factors which were associated with loss of seroprotection during follow-up were examined (9). 283 seroresponders (mean age, 28.6 years; median CD4, 607 cells/mm3) after HBV revaccination were included: 139 having received standard-dose and 144 double dose HBV vaccine. The baseline characteristics were balanced between the two groups. The median follow-up duration was 2.0 and 2.1 years for PWH having received standard-dose and PWH having received double-dose HBV vaccine, respectively. During the follow-up, more PWH in the standard-dose group lost seroprotection than those in the double-dose group (21.0% vs 10.4%, p=0.014). Loss of high-titer seroresponse was more common for the standard-dose group than the double-dose group (41.7% vs 20.0%, p<0.001). Factors associated with loss of seroresponse during follow-up (Table 1) were lower pre-revaccination anti-HBs titer (<2.5 mIU/ml, aHR 14.80 [95% CI, 3.58-61.19], p<0.001) and double-dose HBV vaccine (aHR 0.51 [95% CI, 0.27-0.96], p=0.037).
Table 1: Factors associated with loss of HBV seroprotection
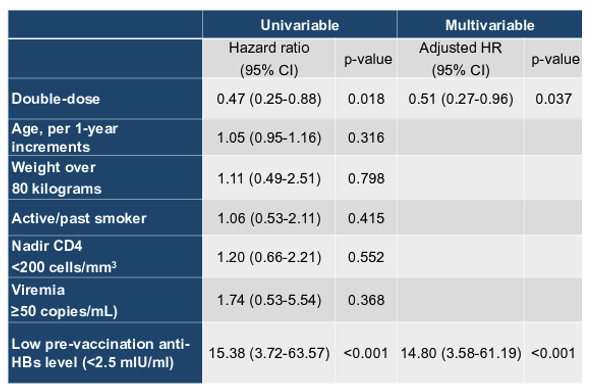
In conclusion, three double-dose HBV revaccination leads to more sustained seroprotection than standard-dose revaccination in PWH. For PWH who fail to achieve high-titer seroresponse after revaccination, more frequent follow-up of anti-HBs titers is recommended to detect the loss of seroprotection for HBV revaccination to be administered timely.
HBV Treatment
At this year CROI, a new and most likely last analysis from the ALLIANCE study was presented (10). 48-week results from the ALLIANCE Study were already published last year (28). Aim of this phase-3-study (ALLIANCE) was to compare outcomes of bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) vs dolutegravir + emtricitabine/ tenofovir disoproxil fumarate (DTG+F/TDF) in participants initiating treatment for HIV-1 and HBV and to examine HBV DNA suppression and predictors of HBs/eAg loss in this coinfected population. Adults with HIV-1/HBV were randomized 1:1 to initiate blinded treatment with B/F/TAF or DTG+F/TDF (with corresponding placebo). The aim of this additional subgroup analysis was to explore factors associated with the HBV treatment response of B/F/TAF versus DTG + F/TDF in adults coinfected with HIV-1 and HBV at Week 96. Of note, there were slightly more individuals with higher baseline HBV-DNA levels in the TDF group as well as more with lower CD4-counts, all well-known factors to impact HBs/HBe-Ag seroconversion rates. Overall, median HBV-DNA decline up to week 96 did not differ between the two groups making differences in antiviral potency over time rather unlikely. Also, initial differences between the group with rate of participants reaching undetectable HBV-DNA lost statistical significance over time. Nevertheless, there were higher HBeAg loss and seroconversion rates in the TAF versus TDF study arm. The initial significant difference in HBs loss was no longer found to be significant at week 96 in the overall population. B/F/TAF however resulted in higher rates of HBsAg loss compared with DTG + F/TDF in the following subgroups: Asian race, Higher study drug adherence (≥ 95%), lower HBV viral load (baseline HBV DNA < 8 log10IU/mL) and HBV genotype B/C (see Table 2.
Table 2: Treatment Difference in Proportion of Participants With HBsAg Loss at Week 96, by Subgroup
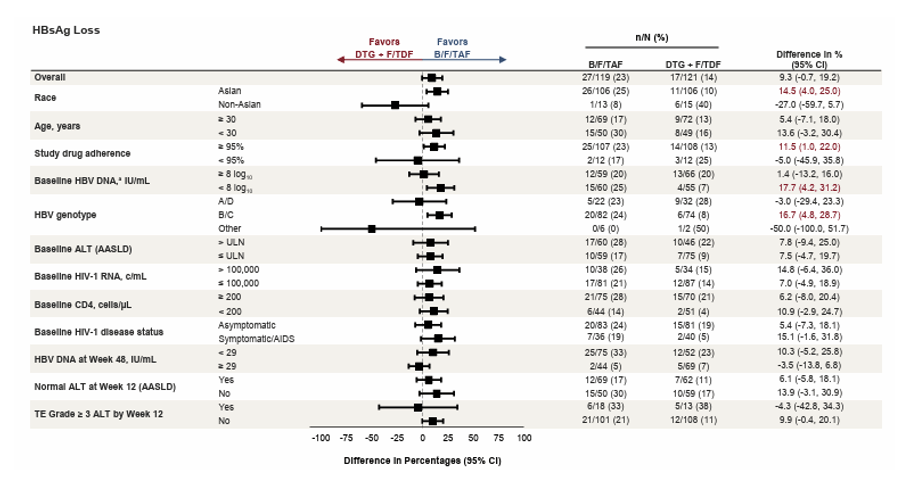
Data are from the serologically evaluable full analysis set for individuals who were HBsAg positive and HBsAb negative/missing at baseline. difference in percentages and 95% CIs were calculated based on the MH proportions adjusted by baseline HBeAg status (positive vs negative) and baseline HBV DNA category (< 8 log10IU/mL vs ≥ 8 log10IU/mL), if not the subgroup factor. aProportion difference and 95% CI from normal approximation without stratification, as not calculable by stratum-adjusted MH method. AASLD, American Association for the Study of Liver Diseases; ALT, alanine aminotransferase; B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; c, copies; DTG, dolutegravir; F/TDF, emtricitabine/tenofovir disoproxil fumarate; HBsAg;hepatitis B surface antigen; MH, Mantel-Haenszel; ULN, upper limit of normal; TE, treatment-emergent.
Consistent with the overall population, B/F/TAF resulted in higher rates of HBeAg loss as well as ALT normalization than DTG + F/TDF in most subgroups. As Hbs/HBe-Ag loss or seroconversion can occur even after more extended follow-up periods a longer follow-up is warranted to see whether these results persist over time.
HBV reactivation after switch to 2DR regimens
Anticore antibody (anti-HBc) in absence of hepatitis B surface antigen (HBsAg) is defined as an isolated anti-HBc status that may be associated with HBV reactivation particularly in the setting of immunosuppression. At this year CROI a study was presented which investigated HBV reactivation in anti-HBc-PLWH (with isolated anti-HBc or with anti-HBc and anti-HBs) after the switch from ART including drugs active on HIV and HBV (tenofovir disoproxil fumarate, TDF and tenofovir alafenamide, TAF) to an anti-HBV sparing regimen (anti-HBVsr) (11). This cohort study included PLWH with anti-HBc (isolated or with anti-HBs-Ab) who switched from NA-containing regimens to dolutegravir/rilpivirine (DTG/RPV) or cabotegravir/rilpivirine long-acting (CAB/RPVLA). Overall, 34 PLWH with anti-HBc and anti-HBs-Ab and 7 with isolated anti-HBc switched to an anti-HBVsr. The median follow-up after switch to anti-HBVsr was 8.91 months [(IQR 6.78- 24.14) overall, and in PLWH with anti-HBs-Ab 8.96 months (IQR 6.78- 24.14) and in PLWH with isolated anti-HBc 6.97 months (IQR 5.56- 43.22); p=0.742]. No reactivations were observed in PLWH with concomitant anti-HBc and HBs-Abs. Of 7 PLWH with isolated anti-HBc, 1 (14.3%) experienced HBV reactivation 3 months after switching to CAB/RPVLA. In this individual a positive HBV-RNA at baseline (8 copies/mL) was observed together with a very low level of HBV-DNA (6 copies/mL) by ddPCR assay (see figure 3).
Figure 3: Trend of HBV-DNA-RNA and serology in the participant with HBV reactivation. Alignment of the partial HBsAg before any ART and during NBV reactivation in the bottom.
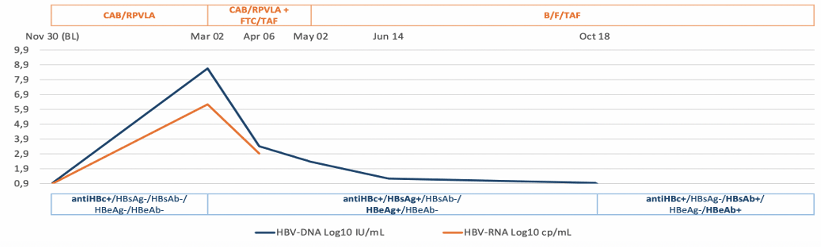
In conclusion, HBV reactivation after switching to anti-HBVsr is unlikely in PLWH with both anti-HBc and anti-HBsAb. Close monitoring of ALT and possibly HBV-DNAi is strongly recommended in PLWH with isolated anti-HBc switching to to an anti-HBV sparing regimen.
A second study investigated whether occult hepatitis B virus infection (OHBVI), indicated by the presence of a reactive anti-HBc-Ab in serum, increased the risk of transaminase elevations, which could indicate hepatitis B reactivation, among PWH who switch to two-drug regimens (2DR) and discontinue tenofovir (TFV) and/or lamivudine (3TC) (12). A total of 167 patients switched to a 2DR containing 3TC (Cohort 1). Among them 33 patients (19.8%) had discontinued TDF and 134 (80.2%) discontinued TAF. 118 switched to a 2DR without TFV and 3TC (Cohort 2), of whom 11 (9.3%) discontinued TDF and FTC, 61 (51.7%) TAF and FTC and 46 (39%) only 3TC. A grade ≥1 LFTI was observed in 20 (12%) of those in Cohort 1 and in 13 (11%) of those in Cohort 2. No grade ≥3 LFTI was observed. Among patients on 3TC-including 2DR, the incidence of grade≥1 LFTI was 4.59 and 7.47 per 100 person years of follow-up in those with and without OHBVI. The time to the event did not significantly differ according to OHBVI (log-rank test P=0.259). Among those with no anti-HBV agents in the regimen (Cohort 2), the incidence of grade ≥1 LFTI was 8.04 and 8.68 per 100 patient-years of follow up, based on the presence or absence of OHBVI. Again rates were not significantly different in the time to the event based on OHBVI status (log-rank test P=0.763). A limitation of the study is lack of HBV-DNA data.
In conclusion, the presence of an OHBVI infection was not significantly associated with transaminase elevation among PWH treated with 2DR lacking anti-HBV agents. This real-life observation provides reassurance regarding the safety of transitioning to dual therapy in patients with OHBVI.
HBV cure
In the context of novel treatment strategies aimed at HBV cure by eliminating or silencing the cccDNA reservoir, its non-invasive evaluation with blood viral biomarkers is critical to monitor intrahepatic viral clearance and guide treatment cessation. One of the caveats of these biomarkers is their ability to accurately distinguish biomarkers expressed from cccDNA versus those expressed from integrated viral sequences. HBsAg can be expressed from viral sequences integrated in the host genome which undermines its value in predicting cccDNA levels and transcriptional activity, particularly in HBeAg(−) patients and in patients under NUC therapy. Circulating HBV RNA (cirB-RNA) concentration is a promising novel biomarker for antiviral treatment monitoring. Quantification of cirB-RNA, measured with research laboratory developed assays, have good predictive power for both on treatment serological response and off-treatment durability. In a wonderful overview on novel HBV markers professor Zoulim also showed a summary slide on the current HBV pipeline and potential combination treatment approaches which look very promising and indicate that many new findings from different combination treatments against HBV can be expected shortly (see figure 4) (7).
.
Figure 4: The HBV drug pipeline and the potential for combination therapy to cure HBV
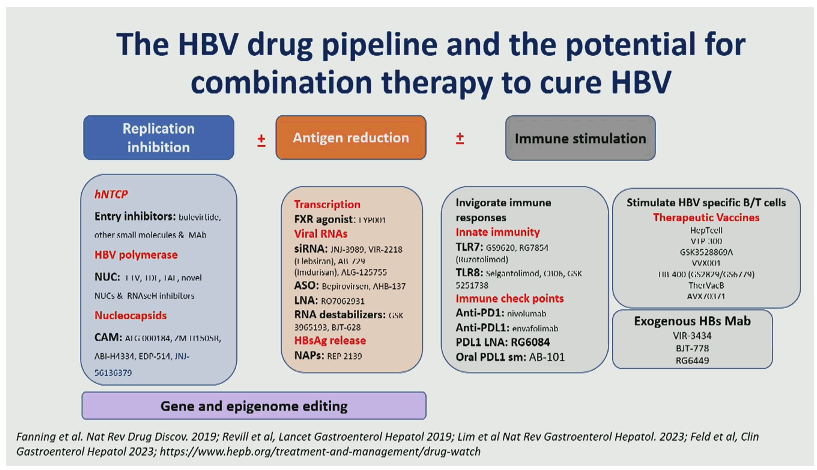
Conclusion: investigational biomarkers are now used for the evaluation of target engagement and antiviral efficacy to assist the development of new antivirals (Capsid Assembly Modulators, SiRNA, antisense oligonucleotides, etc.) and immunomodulatory agents (check point inhibitors, TLR agonists, therapeutic vaccines, etc.). Altogether, these non-invasive viral markers show promise for a deep phenotyping of patients and show potential for patient stratification and novel treatment evaluation.
Hepatitis D pathogenesis
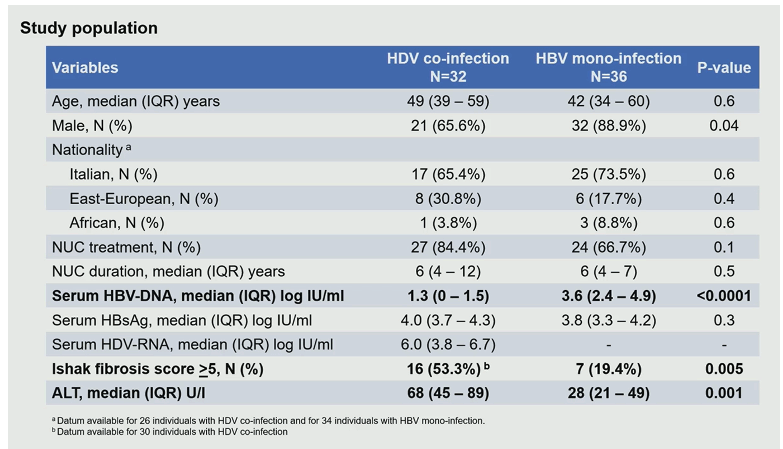
In HBV chronic infection it is well established that HBV-DNA can integrate into the genome of infected hepatocytes. HBsAg thereby can derive not only from covalently circular closed DNA (cccDNA) but also from integrated HBV-DNA. In particular the integrated HBV-DNA represents the major source of HBsAg in the eAg-negative phases of chronic HBV infection. The contribution of HBV integration (as source of HBsAG) to HDV persistence has not yet been studied in vivo in the setting of chronic HDV infection. The aim of this study was to gain new knowledge on the interplay between the two viruses which may play a role in future treatment developments (5). The three aims of this study were to investigate HBV and HDV replicative activity and their interplay directly in the liver, to discriminate the amount of HBsAg derived from cccDNA and from integrated HBV-DNA and their role in sustaining HDV persistence and finally to correlate intrahepatic markers of HBV and HDV activity with peripheral markers currently used in clinical practice (5). Liver tissue was analyzed from 68 HBe-Ag negative individuals (32 with chronic HDV and 36 with chronic HBV monoinfection). The baseline characteristics of the study population are summarized in table 3.
Table 3: Baseline characteristic of the study population
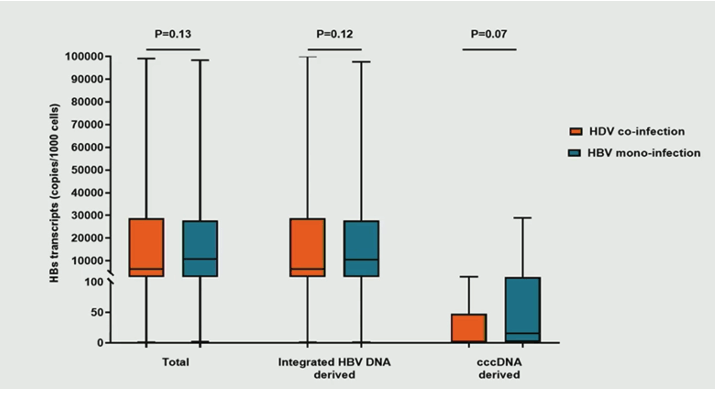
By comparing intrahepatic HBV markers, significant differences in HBV reservoir size were observed between HDV coinfection and HBV mono-infection (Total HBV-DNA, cccDNA and pgRNA were all significantly lower for HDV coinfected subjects). In the overall population, intrahepatic levels of total HBs transcripts positively correlated with serum HBsAg. Notably, by analyzing the source of HBs transcripts the researchers found that >99% of them derived from integrated HBV-DNA, with a very limited contribution of cccDNA transcriptional activity. Despite the limited HBV reservoir, HDV coinfection was characterized by a production of total and integrated HBV DNA-derived HBs transcripts comparable to HBV mono-infection (see figure 5). In line with HBV markers the production of cccDNA-derived HBs transcripts tended to be lower in the setting of HDV coinfection.
Figure 5: Boxplot comparison of intrahepatic levels of HBs transcripts in copy/1000cells between HDV coinfected and HBV mono-infected subjects. Mann Whitney test was used to assess statistically significant differences.
7/32 individuals with chronic HDV coinfection showed no cccDNA or cccDNA-derived HBs transcripts. All of these patients were undetectable for HBV-DNA. Focusing on these 7 individuals an intensive HDV activity was observed both at serum and intrahepatic levels, suggesting that HDV persistence can be sustained by HBsAg derived from integrated HBV-DNA. Overall, these data suggest the existence of independent HBV and HDV replicative pathways. In conclusion, HDV replication pathway acts independently from the size of intrahepatic HBV reservoir and is fueled by an abundant production of HBs transcripts, mainly derived from integrated HBV-DNA. These issues are crucial for defining mechanisms underlying HDV persistence, that could hamper the success of therapeutic strategies.
HDV Prevalence
Infection with Hepatitis D virus (HDV) is a potential complication of hepatitis B infection. HDV infection can be acquired simultaneously with hepatitis B as a coinfection or as a superinfection with chronic hepatitis B. Globally, it is estimated that 4.5 % of people living with chronic hepatitis B are also affected by hepatitis D. HDV seroprevalence studies in the US so far are limited. The objective of this study was therefore to estimate the epidemiological prevalence of HDV infection within the United States using HBsAg-positive specimen remnants obtained from routine clinician requests received at a national laboratory network (13). A total of 2,646 HBsAg-positive specimens were included in the study. Figure 6 shows the HDV AB(+) rates by HHS region.
Figure 6: Seroprevalence of HDV Ab(+) patients in the 10 US Department of Health and Human Health Services (HHS) by Regions.
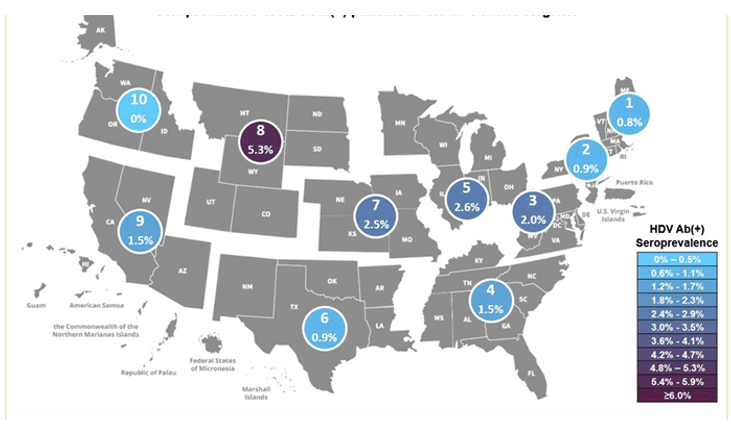
The highest number (n=11) of HDV Ab-positive specimens were identified in HHS region 5, which includes Illinois, Indiana, Michigan, Minnesota, Ohio and Wisconsin. The highest proportion of HDV Ab-positive specimens were identified in HHS region 8. The overall HDV seroprevalence in this study was low with only 1.6%. In the current study almost 40% of HDV Ab(+) specimens were also positive for HDV RNA, supporting the use of confirmatory RNA testing to identify active infection. A limitation is the small number of HDV(+) specimens and the question of how representative the study population was for individuals living with chronic HBV and at risk for HDV coinfection. Further seroprevalence studies are needed to better understand the number of undiagnosed individuals at need for potential treatment interventions.
At this year CROI a study from Taiwan investigated the incidence of HDV infection among HBV-infected PWH and the impact of HDV on mortality and liver-related outcomes in the era of tenofovir-containing ART (14). PWH with HBV-coinfection seeking HIV care between 2011 and 2022 were included and followed until Dec. 31, 2023. Screening for anti-HDV IgG was performed at enrollment and on an annual basis on sequentially archived blood samples. The overall incidence rate of HDV superinfection was 12.65 per 1000 person-years of follow-up (PYFU) after a total of 3873.54 PYFU. An increased number of recent HDV episodes were found in 2016. After a median follow-up duration of 10.2 years (IQR 5.7-12.3), with 86.2% of the total follow-up time covered by tenofovir, the all-cause mortality rate, liver-related mortality rate, and incidence of hepatocellular carcinoma (HCC) were 4.7% (25/534),
1.0% (5/534), and 1.3% (7/534), respectively. HDV-infected PWH had higher rates of liver-related death, cirrhosis, an APRI >2.00 or an FIB-4 >3.25 than those without HDV infection at the end of follow-up (Figure 7). Results were similar when HCV infected patients were excluded.
Figure 7: Outcomes of HDV-infected and HDV-uninfected PWH at the end of follow-up.
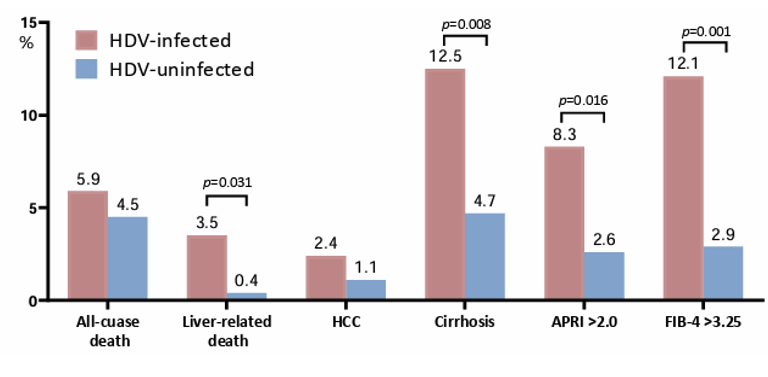
HDV infection was associated with liver-related death in HBV-infected PWH. The risks of all-cause mortality and development of HCC were similar in HBV-infected PWH with and those without HDV infection. In conclusion, in the era of tenofovir-containing ART, PWH with chronic HBV infection remain at risk for HDV superinfection, and HDV infection is associated with liver-related death.
HDV Treatment
At this year's poster session, the results from a multicenter, open-label, randomized, Phase 3 study (NCT03852719) on treatment of HDV with bulevirtide were presented which also included a few HIV coinfected subjects (15). The study was conducted in 16 sites across 4 countries (Germany, Italy, Russian Federation, and Sweden). Patients with chronic HDV were treated with bulevirtide a novel, first-in-class entry inhibitor of both HBV and HDV for 96 weeks with either 2mg or 10mg subcutaneously daily. A delayed treatment arm served as control. The study design is shown in figure 8.
Figure 8: Study Design
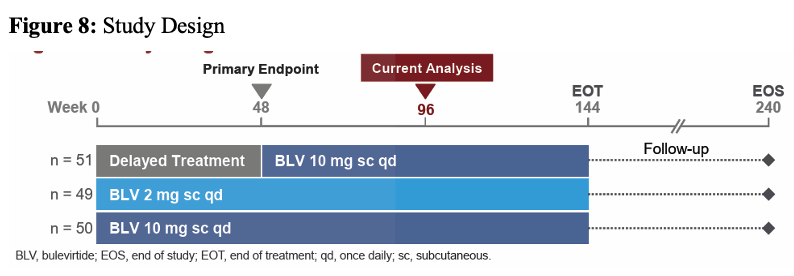
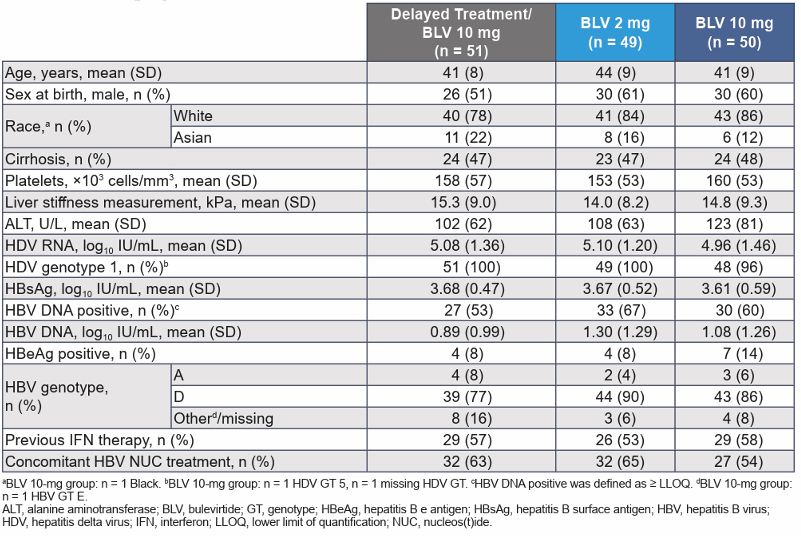
Key inclusion criteria were individuals with chronic hepatitis delta without or with cirrhosis and Child-Pugh-Turcotte score ≤7, an Alanine aminotransferase (ALT) >1× to <10× upper limit of normal and platelets ≥60,000 cells/mm3. PWH and controlled HIV coinfection were allowed into the study (defined as CD4 cell count >500/mL, HIV RNA < limit of detection for ≥1 year). Nucleos(t)ide analogue therapy was permitted for those meeting HBV and HIV guideline criteria. The primary efficacy study endpoint was the proportion of patients achieving a combined response at week 48 and defined as meeting both criteria: Undetectable HDV RNA or decrease by ≥2 log10 IU/mL from baseline and ALT normalization. Table 4 summarizes the main baseline characteristics for the different study arms.
Table 4: Demographic and disease characteristics of the total study population
The main efficacy results up to week 96 are shown in figure 9.
Figure 9: Interim efficacy analysis at week 96
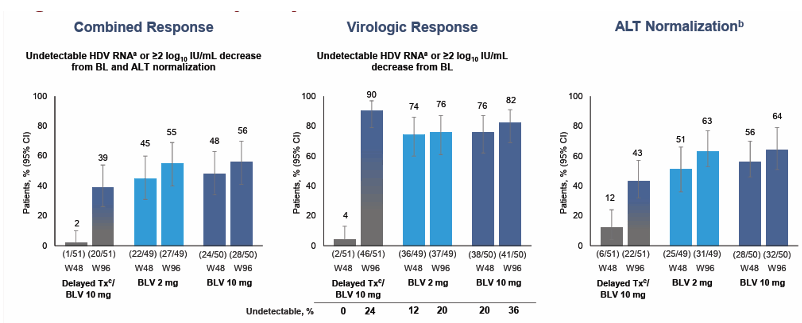
At week 96, 39% to 56% of patients responded with substantially reduced levels of HDV RNA and with normal levels of the liver enzyme alanine aminotransferase, a key marker of liver health. Through 2 years of treatment, bulevirtide was safe and well tolerated. There were no discontinuations of bulevirtide because of adverse events. Two people were included who were virally suppressed for HIV when starting treatment with bulevirtide for chronic HBV/HDV. Both of these people living with HIV had favorable HDV responses with bulevirtide, and bulevirtide had no impact on HIV treatment.
Hepatitis C Long-acting Therapy
Challenges with modern HCV DAA-based therapy include beyond cost and access issues, medication adherence and loss of patients to follow-up, A long-acting injectable (LAI) for treatment of hepatitis C providing 8 weeks of pharmacokinetic exposure from a single administration would overcome many of these challenges by providing a one-shot cure. Glecaprevir (G) and pibrentasvir (P) possess key physicochemical and pharmacokinetic properties shared by other LAI paradigms. At CROI a study was presented where G and P were co-formulated as a fixed dose combination LAI (250 mg/mL G, 250 mg/mL P (3). Dosing volumes of 0.075, 0.15 and 0.3 mL (representing active doses of 18.75, 37.5 and 75 mg) were administered to male Sprague Dawley rats (n = 4, 250-300 g) via intramuscular injections to the thighs. The rat plasma PK results for the different drugs and coformulations are shown in figure 10.
Figure 10: Glecaprevir, Pibrentasivir and coformulated G/P intramuscular long-acting injectable rat plasma PK results
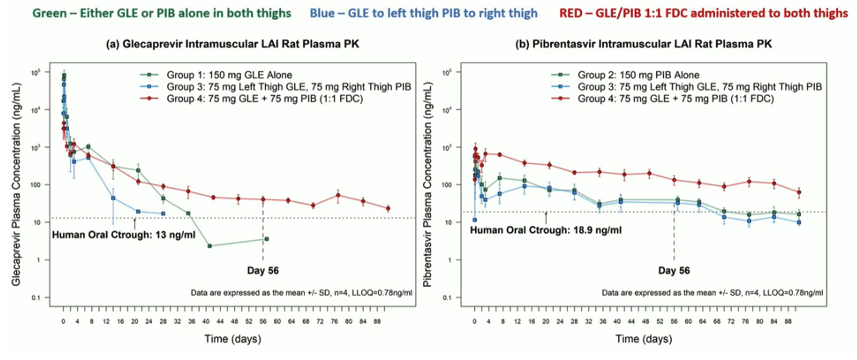
The combination of both drugs (G/P) within an FDC maintained a longer half-life for glecaprevir and improved exposure to pibrentasvir, maintaining plasma concentrations above the human oral ctrough.
Conclusion: Preclinical data for a novel G/P LAI demonstrate sustained therapeutic concentrations in rats over a period of 13 weeks, exceeding the 8-week human target duration. Parenteral administration did not adversely affect hepatic penetration in comparison to the oral route. No adverse effects were observed but GLP-toxicology studies are planned to support clinical translation.
HCV Elimination
Advances in HIV and hepatitis C virus (HCV) prevention and treatment have led to plans to end the HIV epidemic and achieve HCV elimination by 2030. Data on long-term trends in the uptake of combination HIV/HCV prevention services and their impact on HIV/HCV incidence among people who inject drugs (PWID) are limited. The AIDS Linked to the IntraVenous Experience (ALIVE) study is a community-based cohort of PWID aged ≥18 years in Baltimore, Maryland with 5 enrollment periods: 1988-89, 1994-95, 1998, 2005-08, and 2015-18 (4). The study assessed trends in HIV and HCV seroincidence, prevalence of injection practices, self-reported use of prevention and treatment services, HIV viremia (>400 c/ mL) and HCV viremia (>500 IU/mL) using Poisson and logistic regression with generalized estimating equations (4). Data were censored at 12/31/2019 (prior to the COVID-19 pandemic).
Overall prevalence of active injection drug use declined from 89% in 1988 to 36% in 2019 (p<0.001). In the same observation period among PWID, the prevalence of attending syringe service programs increased from 36% in 1988 to 51% in 2019 (p=0.004). Among all participants with HIV the prevalence of any ART use increased from 55% in 2006 to 96% in 2019 (p<0.001). Among participants with chronic HCV infection, the prevalence of ever being treated for HCV increased from 2% in 2014 to 56% in 2019 (p<0.001). The seroincidence for HIV and HCV is shown in figure 11 over time.
Figure 11: Seroincidence for HIV and HCV.
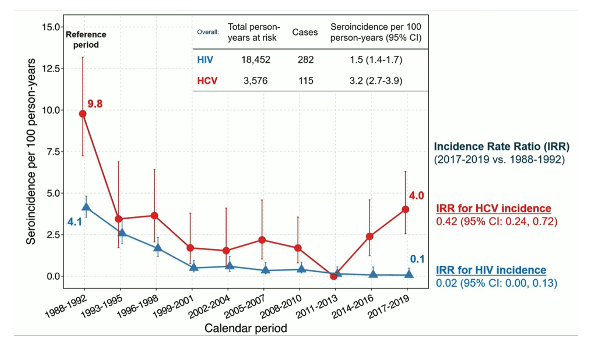
Conclusion: In the ALIVE cohort, HIV and HCV seroincidence decreased over time in PWID corresponding with increased prevention efforts and behavioral changes. HCV seroincidence however, remained high. Intensified efforts are needed to achieve HCV elimination among PWID.
Another study addressing HCV elimination in the poster session was from Australia (16). In Australia an estimated 2000-2500 people were living with HIV-HCV co-infection prior to availability of HCV direct-acting antiviral (DAA) treatments (predominantly gay and bisexual men). Rapid DAA scale-up occurred from 2016 following unrestricted DAA access for adults. The aim of this analysis was to evaluate progress towards HCV elimination among people with HIV in Australia. The CEASE prospective cohort study enrolled people with HIV and anti-HCV antibodies, irrespective of HCV RNA status, from 14 primary and tertiary clinics. Enrolment (ENR; 2014-2016), Follow-Up 1 (FU1; 2017-2018) and Follow-Up 2 (FU2; 2021-2023) visits were undertaken (16). Following rapid DAA scale up, HCV RNA prevalence declined from 84% to less than 1% by study end. HCV reinfection incidence also declined over time. Death incidence remained stable. Figure one shows the HCV status over time for the different follow-up visits. The plot visualizes transitions between HCV status and states over the study period.
Figure 12: Alluvial plot of HCV status. Legend: HCV RNA +, hepatitis C virus RNA detected; HCV RNA -, hepatitis C virus RNA not detected; DEATH; deceased, NOT AVAIL , no HCV RNA test available.
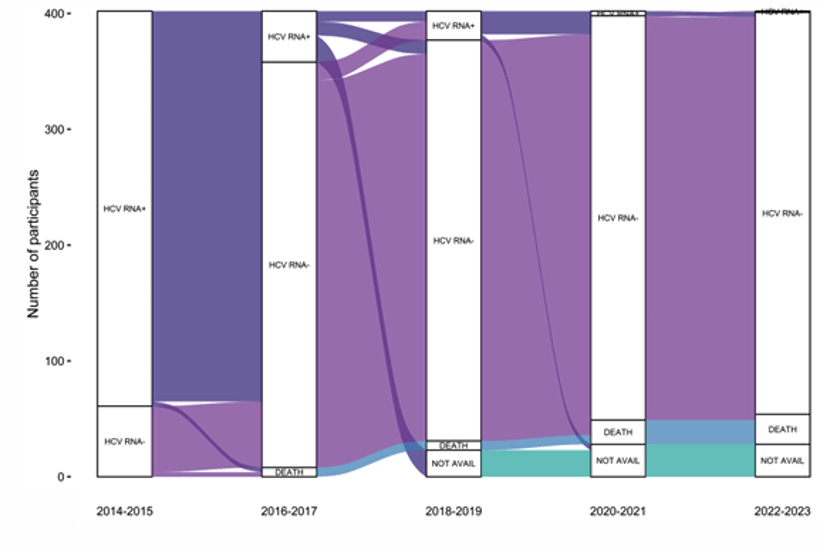
Conclusion: HCV prevalence among people with HIV in Australia has declined substantially following rapid DAA scale-up and reinfection incidence has declined. Surveillance for HCV (re)infection among those people with HIV and HCV-risk behaviors should be continued. Despite universally high treatment success, death rates within this cohort remain significantly higher than the age matched general population and are likely related to chronic co-morbidities and lifestyle factors as well as underlying liver disease.
An important exercise in achieving HCV elimination is to look at the vulnerable populations which are remaining viremic despite broad introduction of DAA therapy to better identify strategies for linking those individuals for therapy. National US data on the burden and risks for active hepatitis C (HCV) infection in people with HIV (PWH) during the direct-acting antiviral (DAA) era are however limited. At CROI a poster was presented which evaluated prevalence (2011-22) and risk factors (2018-22) for HCV viremia in PWH to understand current progress and guide future efforts toward HCV micro-elimination in PWH (17). The study was conducted at 9 sites of the CFAR Network of Integrated Clinical Systems (CNICS) cohort and performed a cross-sectional examination of HCV viremia prevalence of adult PWH in clinical care (≥1 encounter) for each time period and demographic group: Time period: 2011-13: Pre-Interferon Free DAA Era, 2014-17: Early DAA Era, 2018-22: Current DAA Era. The demographic groups included age, gender, race/ethnicity, HCV viremia: (positive HCV RNA or genotype on most recent lab result). The prevalence of HCV viremia among PWH in the US by DAA era and demographic group is shown in table 5.
Table 5: Prevalence of HCV viremia among PWH in the US by DAA era and demographic group. For each cell, n is the number of positive tests, and % is the proportion of positive tests within a cell's group (e.g., n=77 or 3.0% of <30 year old PWH had a positive HCV RNA or genotype on their last test in 2011-13).
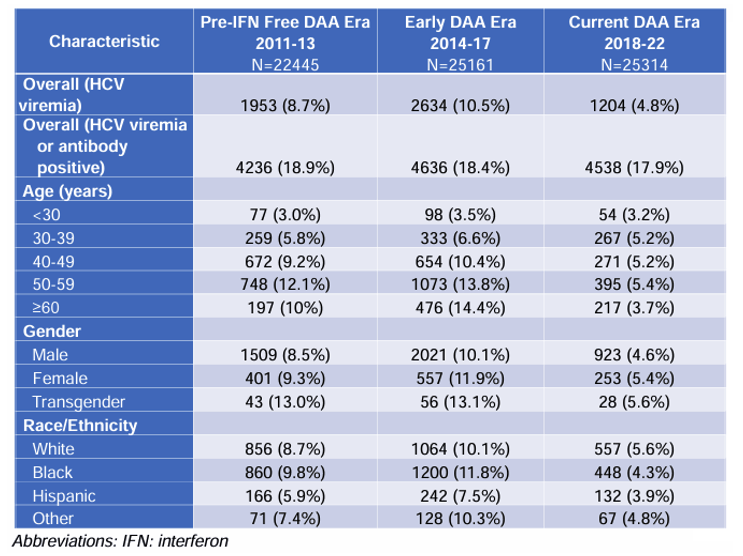
The prevalence of HCV viremia during the DAA era in this US-based national cohort of PWH improved over time and across demographic groups but remains much higher than those without HIV (29). The risk estimates of risk factors for HCV viremia in the current DAA era is depicted in figure 13.
Figure 13: Relative risk estimates of demographic, clinical, and behavioral factors
for HCV viremia in the current DAA era (2018-22).
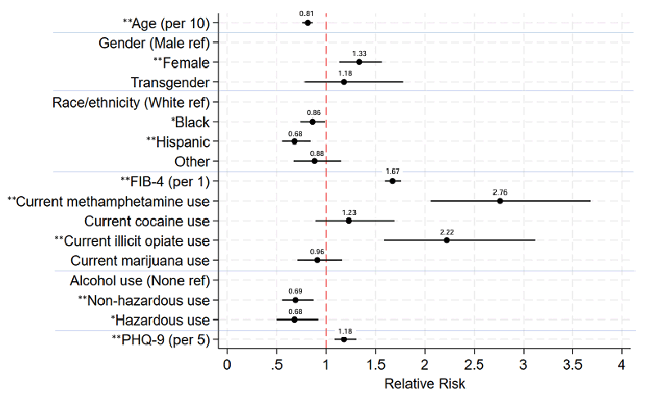
aModel adjusted for CNICS site and listed covariates.
*P<0.05, **P≤0.001
Non-hazardous alcohol use defined as AUDIT-C score 1-4 in men and 1-3 in women.
Hazardous alcohol use defined as AUDIT-C score >4 in men and >3 in women
The disparity (range) in HCV viremia prevalence across age, gender, and race/ethnicity groups was smaller in 2018-22 than earlier time periods. Active drug use was still an independent risk for remaining viremic. These findings underscore the continued importance of prioritizing HCV testing and treatment in all PWH, especially among certain key groups, and potential benefits of addressing substance use and mental health for achieving HCV elimination goals.
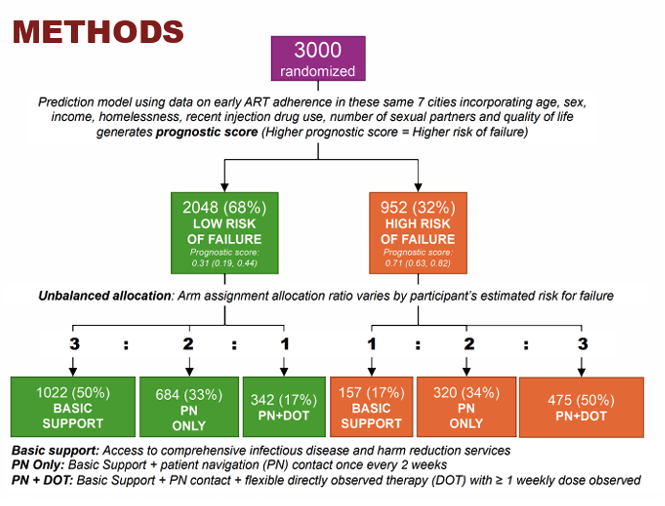
HCV elimination is unlikely without curing people who inject drugs (PWID). Many PWID will however need additional support to achieve HCV cure, but resources are limited. At CROI in the HCV elimination poster session results from the STOP-C trial were presented which evaluated whether HCV treatment outcomes could be optimized by tailoring adherence support to PWID need across community-based HIV prevention and treatment centers in 7 cities across India (18). STOP-C was a precision-randomized trial: arm assignment probabilities varied by estimated probability of treatment failure (Figure 14). Eligibility criteria included history of injection and no prior HCV treatment; decompensated cirrhosis was excluded.
Figure 14: Study design
2798/3000 (93%) completed SVR assessment. ITT analysis for SVR resulted in an overall SVR of: 49% high risk; 63% low risk; No difference by arm in high risk group. There was a significantly higher SVR for PN+DOT vs. basic support in the low risk group (aRR: 1.10, p=0.04). In the per protocol analysis overall SVR was 60% in the high risk group and 70% in the low risk group. A significantly higher SVR was obtained for PN+DOT vs. basic support in the low risk group (aRR: 1.11, p=0.02). SVR increased from 36.1% in the highest risk quartile receiving PN only to 76.5% in the lowest risk quartile receiving PN+DOT (see figure 15).
Figure 15: SVR by prognostic score and arm
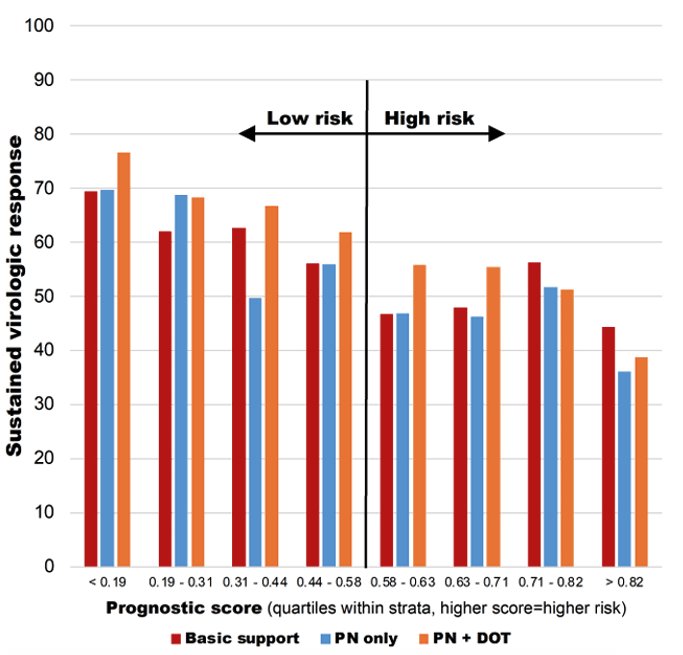
In summary, SVR in this population of PWID who were young and actively injecting was disappointingly low. Among those at lowest risk of failure, highly intensive adherence support improved outcomes. Among those at highest risk of failure, none of the adherence support interventions had any impact suggesting that additional interventions beyond adherence support (e.g., housing support, long-acting MOUD) may be needed to achieve optimal SVR in this group.
Recently acquired HCV
Numerous studies have previously tried to shorten treatment duration in PWH experiencing an episode of recently acquired hepatitis C. Most of these all-oral DDA based shorter treatment schemes did not work out at least in individuals with high baseline HCV viral loads. Arthur Kim from Boston presented the results of a study shortening HCV DAA therapy to a single month (19). Indeed, simplified treatment schedules may further facilitate the national plan of HCV elimination. A5380 (PURGE-C) was a prospective, phase II, single-arm multicenter trial evaluating the efficacy and safety of once daily oral glecaprevir/pibrentasvir (G/P) for 4 weeks in adults with early HCV. Early HCV was defined as new ALT elevation (≥5x ULN or >250 U/L if normal ALT in prior year, or ≥10x ULN or >500 U/L if no or abnormal ALT in prior year); or detectable HCV RNA with prior negative antibody (1st infection) or HCV RNA (re-infection) within 24 weeks prior to study entry. The primary study endpoint was SVR12, defined as HCV RNALLOQ (lower limit of quantification) at 12 weeks after treatment cessation.
45 enrolled subjects completed HCV treatment. Treatment with 4 weeks of glecaprevir/pibrentasvir for early HCV infection resulted in a SVR12 (cure) rate of 84%. Six participants demonstrated recurrent viremia after end of-treatment at or before SVR12 determination. Most of them had a high HCV viral load at baseline suggesting in line with previous studies that in recently acquired HCV shorter treatment durations may not work equally well in high baseline HCV RNA levels (see figure 16). Obviously, reinfection also needs to be considered. Further phylogenetic analyses are on their way.
Figure 16: Baseline HCV RNA by Outcome
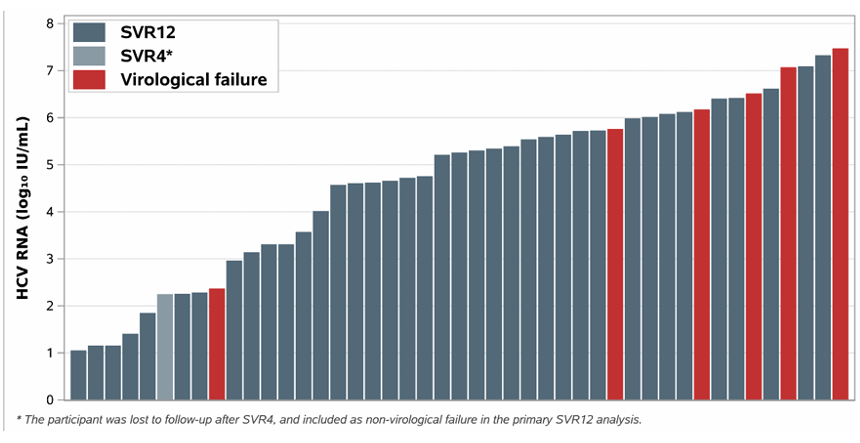
Conclusion: As simplified treatment approaches are critical for HCV elimination, a 4-week G/P regimen could be tested in implementation programs aiming to cure early HCV at least in low viremic subjects. Cure with 4-week regimens will also help to inform strategies for the deployment of long-acting injectable antivirals for HCV.
HCV Reinfection
HCV reinfections either after spontaneous clearance or sustained virologic response (SVR) pose a challenge in HCV elimination, particularly in high-risk populations such as MSM or intravenous drug users. In the poster session a study from Thailand determined the incidence and tested risk factors of HCV reinfection in a longitudinal early-treated acute HIV cohort from Thailand (20). RV254/SEARCH010 participants aged ≥ 18 years who initiated antiretroviral therapy (ART) within days during acute HIV infection (AHI) were enrolled into the study. Between 05/2009 and 08/2023, 127/724 (17.5%) participants were diagnosed with HCV infection of whom 18 spontaneously cleared, 73 achieved SVR and 14 (15.4%) developed HCV reinfection, all were MSM with a median age of 34.0 (IQR 29.0-37.0) years. The risk factors associated with HCV reinfection are listed in table 6.
Table 6: Factors associated with HCV reinfection
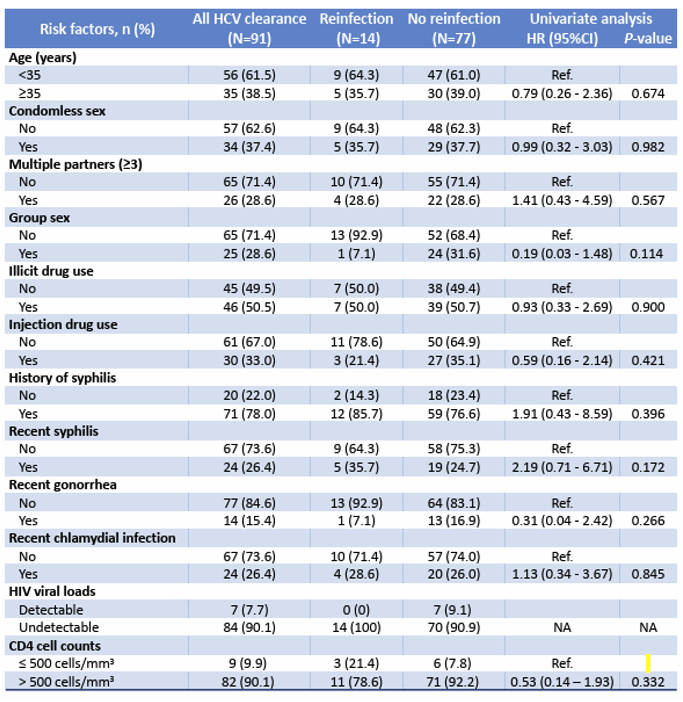
No significant associations between risk factors and HCV reinfection were identified int this study. In summary, in this early-treated AHI cohort of Thai MSM, there is a high incidence of HCV reinfection with a short median time to reinfection of 1.3 years. No significant associations between risk factors and HCV reinfection were identified in this study. Post-clearance follow-up with counseling and preventive strategies to reduce ongoing risk behavior are needed to reduce HCV reinfection.
Additional data on HCV reinfection was presented from the MINMON trial (21). The MINMON (ACTG A5360) trial previously demonstrated that a minimal monitoring approach for HCV treatment with sofosbuvir velpatasvir (SOF/VEL) was safe and efficacious in a diverse global population (30). This new analysis used phylogenetics to estimate HCV re-infection rates and resistance associated substitutions (RASs) among participants with post-treatment HCV RNA > lower limit of quantification (LLoQ) (19). Of all participants, 17 had re-infection during 438 person-years of follow-up (re-infection rate 3.9 per 100 PY [95% CI 2.4-6.2]). Of 17 re-infections, 12 were from HCV RNA collected at week 48 or 72. All 17 participants with HCV re-infection were assigned male sex at birth (13 endorsing MSM), 15 living with HIV, 14 had baseline genotype 1a, and 13 were from Thailand (of whom 11 were MSM with HIV and genotype 1a). At baseline, VEL-specific RASs were detected in three of the 12 participants (25%) who experienced HCV treatment failure and in 43 of 385 participants (11%) who either achieved SVR or were reinfected. In conclusion, considering re-infections, SVR observed in MINMON was 96.5%. The high HCV reinfection rate even prior to SVR, especially among MSM living with HIV underscores the need to scale-up evidence based interventions to reduce re-infection while simultaneously increasing screening and treatment to minimize HCV viremic burden in the population.
Liver disease outcomes following DAA HCV cure in PWH with advanced fibrosis
The outcome of liver disease in PLWH and HCV coinfection following DAA-induced HCV cure is still not well defined. A study from Spain was presented at CROI which analyzed prognostic factors for HCV-related compensated advanced chronic liver disease (cACLD) in persons with HIV (PWH) who achieved sustained viral response (SVR) with direct-acting antivirals (DAA) (22). The researchers also explored baseline liver-stiffness measurements (LSM) and posttherapy LSM based risk stratification models to predict outcomes. This multicenter (21 sites) retrospective study of PWH with HCV-related compensated advanced chronic liver disease (ACLD) included persons who achieved SVR after DAA therapy in Spain between 2014 and 2017. The study cohort included 1,145 patients with cACLD: 384 (33.5%) with advanced fibrosis and 761 (66.5%) with compensated cirrhosis. After a median follow-up of 40.9 months, incidence rates of events are shown in Table 7.
Table 7: Frequency and incidence rate events in PWH with cACLD following DAA HCV cure.
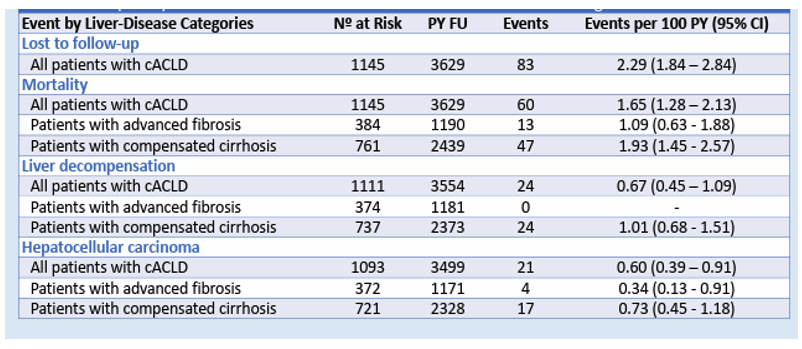
Baseline LSM and Semmler's LSM/Platelet index (29) after therapy showed good predictive ability for decompensation. In the absence of cofactors, patients with a baseline LSM ≤ 13.5 kPa or with an LSM <12 kPa and platelet count >150x10^9/L after therapy may not need ongoing surveillance for portal hypertension, including LSM and endoscopy. The utility of LSM as a predictive tool for decompensation over 42 months post-therapy is shown in Figure 17.
Figure 17: Utility of LSM as a predictive tool for decompensation in PWH with cACLD HCV cure over 42 months post-therapy assessed using t-ROC curves.
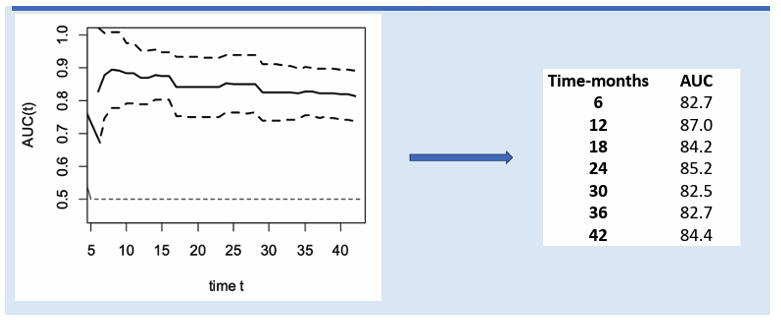
This study enhances understanding of decompensation and HCC risk post-DAA therapy in PWH with HCV-related cACLD.
HCV DAAs and breast milk
Treatment of hepatitis C virus in the postpartum period is delayed until after the completion of breastfeeding due to lack of data on infant exposure and safety. At CROI a study was presented which investigated breast milk transfer of sofosbuvir (SOF), GS-331007 (SOF inactive metabolite) and velpatasvir (VEL) in postpartum women being treated for HCV with SOF/VEL who were not intending to breastfeed (23). Women undergoing postpartum HCV treatment with SOF 400mg/VEL 100mg daily for 12 weeks and willing to provide pumped breast milk were eligible for the study. SOF/VEL treatment was started within 36 hours of delivery and the first PK visit took place within 24 hours of treatment start. Paired maternal blood and breast milk were obtained at 2 or more PK visits. VEL, SOF and GS-331007 concentrations in plasma and breast milk were measured using validated UPLC-MS/MS assays (LLOQ 5 ng/mL for VEL in plasma and breast milk and SOF/007 in plasma; 1 ng/mL for SOF/007 in breast milk). The infant daily dose was calculated using drug concentrations in
the milk and considering that the amount of milk ingested by an exclusively breastfed infant is 150 mL/kg/day. Four participants were enrolled with a median (range) age of 29.5 years (27-36); 3 white, 1 multiple races; all were multiparous with median (range) weight of 69 kg (58-88) and median creatinine of 0.5mg/dL range (0.5-0.6). Figure 18 shows comparison of sofosbuvir, GS-331007, and velpatasvir breast milk concentrations vs. plasma and vs. time since treatment initiation. Each participant had 2 to 7 PK visits each yielding a total of 17 paired samples. Samples were collected a median (range) time of 1.99 days (0.19-6.13) after treatment initiation.
Figure 18: Comparison of sofosbuvir, GS-331007, and velpatasvir breast milk concentrations vs. plasma (top row) and vs. time since treatment initiation (bottom row).
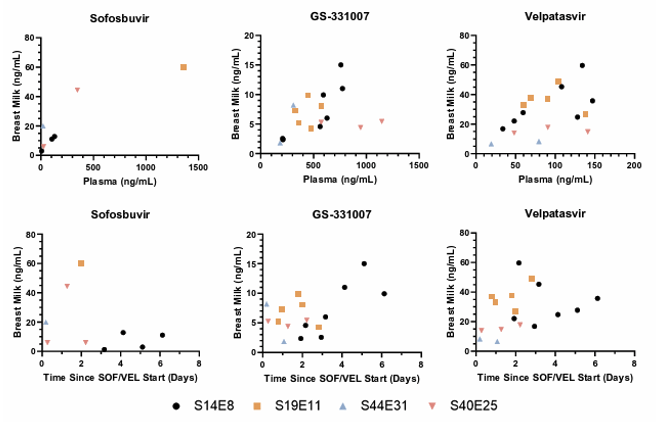
The estimated infant daily dose from breastmilk is less than 0.7% of the daily dose in an adult and a child adjusted to weight. Exposure to sofosbuvir and velpatasvir via breastmilk is minimal, thus is reassuring for infant safety and with low potential for development of HCV resistance with perinatal transmission.
What is new in HCV screening?
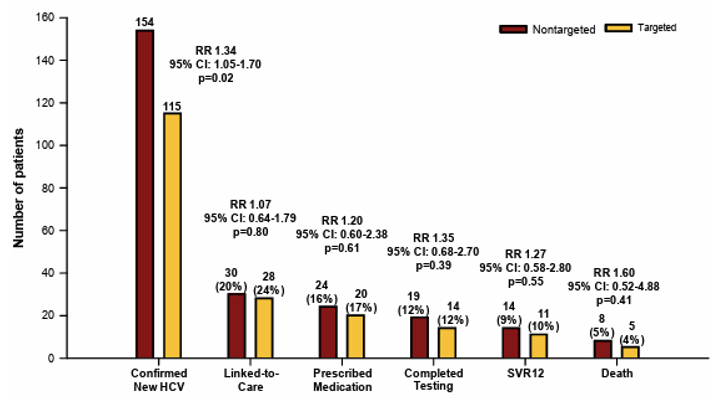
Testing for hepatitis C is the first step toward ultimately curing HCV infection and preventing transmission. Emergency departments (EDs) are key clinical settings for screening given that they serve at-risk patients who commonly do not access healthcare elsewhere. In the HCV poster session, a study was presented which aimed to evaluate the effectiveness of HCV screening in EDs (24). The study was conducted as a prospective multicenter randomized pragmatic trial in EDs in Baltimore, MD, Denver, CO, and Jackson, MS. All patients ≥18 years of age presenting to the ED for care were candidates for inclusion. Patients with critical illness, inability to consent, prior HCV diagnosis, and estimated length of stay <60 minutes were excluded. The eligible patients underwent concealed randomization as part of routine care to either NONTARGETED screening, where all were offered HCV testing using opt-out consent, or TARGETED screening, where all were asked risk questions and any affirmative response led to an offer for HCV testing. Screening was fully integrated into ED care using opt-out consent. Antibody positive tests were followed by reflex RNA testing. Primary outcome was newly diagnosed HCV (RNA+) and elements of the HCV care continuum through 18 months of follow-up. Enrollment occurred from 11/19/2019 through 8/4/2022 with 18-month follow-up completed on 1/31/2024, resulting in 147,498 patients randomized with excellent balance. NONTARGETED Screening: 73,847 allocated, 22.5% accepted testing and 59.5% completed testing, resulting in 154 (1.6%) new diagnoses. TARGETED Screening: 73,651 allocated, 31.9% identified as high risk, 30.4% accepted testing, and 65.3% completed testing, resulting in 114 (2.5%) new diagnoses. Nontargeted screening was significantly associated with new HCV diagnoses when compared to targeted HCV screening (see Figure 19). No differences were identified across the HCV care cascade.
Figure 19: Hepatitis C care cascade within 18 months for newly‐diagnosed emergency department patients, The DETECT Hep C Screening Trial.
Abbreviations: HCV, hepatitis C; RR, risk ratio; CI, confidence interval; SVR12, sustained virologic response 12 weeks after finishing treatment.
In conclusion, nontargeted HCV screening was superior to targeted HCV screening for identifying newly diagnosed HCV in the ED. The significant decay from diagnosis to SVR12 suggests need for innovative models of HCV linkage-to care and treatment from the ED. Screening in emergency departments can play an important role on the path to HCV elimination.
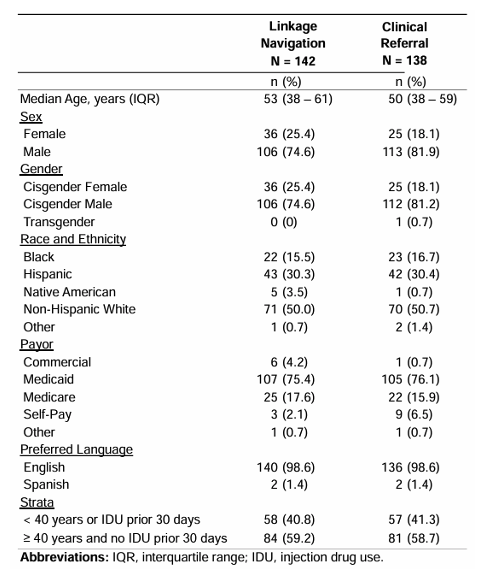
In the same session an additional sub-study from the Detect HCV trial was presented (25). The study aimed to test the effectiveness of linkage navigation (LN) and clinician referral (CR) among ED patients identified with untreated HCV. Further sub-analyses examined the impact of linkage navigation (LN) on individuals less likely to link-to-care (<40 years or recent injection drug use [IDU]). Study population consisted of all patients ≥18 years of age seen in the Denver Health medical center ED with evidence of untreated HCV (RNA+. The Participants were randomized in concealed fashion to CR alone or CR plus LN. Individuals in the CR arm were educated about HCV by their primary ED clinician and given information to access HCV care verbally and in discharge instructions. Individuals in the LN arm met with linkage navigator in the ED or by phone after the visit. LN reiterated basic HCV education, helped schedule and facilitate HCV treatment appointments, and followed up with patients regarding completion of care steps. Enrollment occurred from November 2019 through January 2023. Outcomes were followed through January 2024 (12 months from date of enrollment). 280 individuals were randomized, balanced in baseline characteristics, including individuals <40 years of age or with recent drug use (see Table 8)
Table 8: Demographics and baseline characteristics of emergency department patients with confirmed hepatitis C (HCV) enrolled in The DETECT Hep C Linkage to-Care Trial, N = 280.
Linkage navigation (LN) plus clinician referral (CR) was superior to CR alone for initiating and completing HCV treatment (see figure 19 a). LN plus CR led to 53 (37%) linked to care; 39 (27%) initiated HCV treatment; 29 (20%) completed treatment; 17 (12%) attained SVR12. For CR alone: 25 (18%) linked to care; 18 (13%) initiated HCV treatment; 15 (11%) completed treatment; 9 (7%) attained SVR12. Among individuals <40 years or with recent IDU, there was no significant difference between linkage strategies (see figure 19 b).
Figure 19 a and b: a) Linkage outcomes 12 months after enrollment (all participants). b) Linkage outcomes 12 months after enrollment (participants with recent injecting drug use or age <49 years).
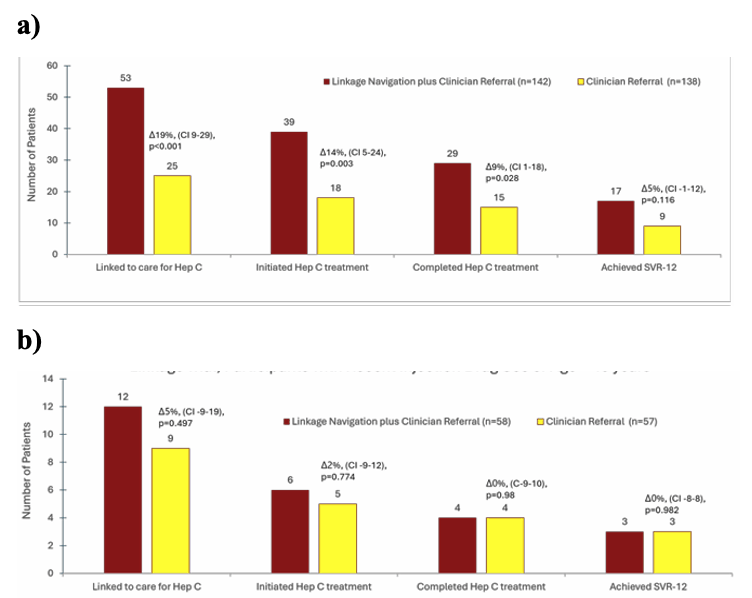
In conclusion linkage navigation combined with clinician referral was superior to CR alone for initiating and completing HCV treatment. Among a subset of individuals <40 years or with recent IDU, there was no significant difference between LN plus CR vs CR alone indicating that for special populations at risk different interventions may be needed.
Migrants born in intermediate and high HBV and HCV-prevalence countries are likely to be at an increased risk for HBV and HCV infection. Data on HCV and HBV prevalence in migrants however, in many countries are limited. At CROI a study from Italy was presented which targeted migrants in Italy with a screening and linkage-to-care programs (26). For this purpose, a prospective, multicenter, study based on the long-term active cooperation between two 3rd level units of Infectious Diseases and four 1st level clinical centers in southern Italy (Naples and Caserta) was designed. The study started in June 2018, was stopped in February 2020, and was resumed in February 2021 until November 2021. All migrants > 18 years old consecutively evaluated for clinical consultation at one of the first-level centers were enrolled.
An anonymous serological screening was offered to seek HIV, HBV and HCV status. The participants who were positive for a virus hepatitis infection and or for HIV were referred for linkage to care at one of the tertiary units. Overall, 3,501 migrants were approached in the study period. 3,417 (97.6%) agreed to be screened. Of the 3,417 subjects screened 185 (4.7%) were anti-HCV-positive, 334 (10%) were HBsAg positive, 61(1.7%) HIV Ab positive. Of the 334 HBsAg positive subjects. 116 (55%) had HBV DNA over than 2000 UI/ML. Of the 116 subjects with HBV DNA over than 2000 UI/ML, 111 (96%) had chronic hepatitis, 3 (2%) had cirrhosis and 2 (1.7%) had HCC; all subjects with HBV DNA over than 2000 UI/ML were linked to care but 3 (2%) were lost to follow up. Eight subjects (2%) were HDV-Ab positive, but only one was HDV RNA positive and linked to care. Of the 185 HCV Ab positive subjects, 53 (29%) were HCV-RNA-positive. Of the 53 HCV-RNA-positive-subjects, 48 (90%) were linked to care, 5 (10%) refused to attend further services. Of these 48, 16 (33.3%) had HCV genotype1b, 11 (22.9%) genotype 1a, 16 (33.3%) genotype 3, 3 (6.3%) genotype 4 and 2 (4.2%) genotype 2. All the 48 HCV-RNA-positive patients started DAA-regimen with sofosbuvir/velpatasvir and completed the 12 weeks of treatment. Of these 48 subjects, 47 (97.9%) showed a sustained virologic response (SVR) at 12 and at 24 weeks after treatment. Only one subject dropped-out during follow-up after finishing the DAA treatment. Comparing patients with HCV Ab positive to patients HCV negative at univariate analysis there were significant differences with regard to number of roommates, history of drug addiction, previous surgery, Intramuscular injection and piercing, respectively p= 0.026, p <0.0001, p=0.019, p=0.001, p=0.010 (see table 9).
Table 9: Comparison of risk factors for HCV between HCV-Ab positive and negative migrants
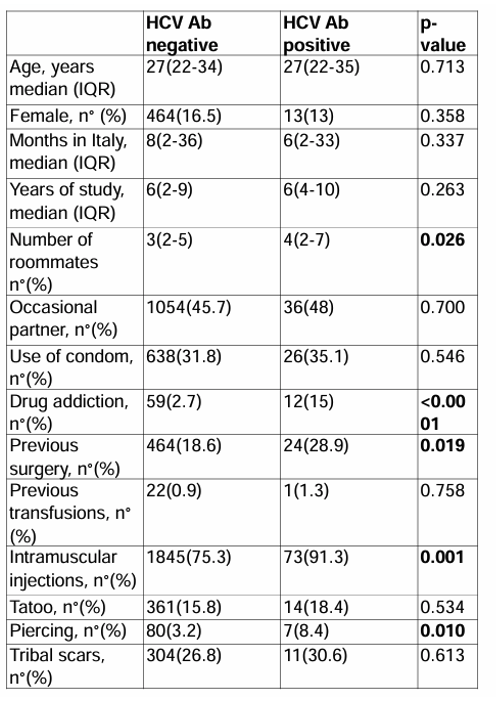
In summary, following an educational phase on the route of transmission and treatment availability for HIV, HBV and HCV nearly 98% of subjects agreed to be screened and evaluated for HIV and/or hepatitis virus infection. Interventions like this appear useful for the viral hepatitis and HIV screening as well as linkage to-care and treatment in a difficult to manage population. More data on usefulness and cost-effectiveness of screening program in these vulnerable populations are needed.
HIV and steatotic liver disease
Steatotic liver disease (SLD) is a rising cause of liver-related morbidity and mortality worldwide, including among persons with HIV (PWH). In the liver case workshop on Sunday, the new nomenclature for SLD was introduced by Professor Price, which is now referred to as predominantly metabolic dysfunction-associated (MASLD) and can overlap with alcohol-related liver injury and is replacing the previous nomenclature NAFLD (see figure 20) (8). This new definition will clearly help to remove some of the stigmatizing language from the old nomenclature but also allow a diagnosis on more stringent diagnostic criteria.
Figure 20: New nomenclature for steatotic liver disease (adapted from Rinella ME et al, Hepatology, 2023 (31)).
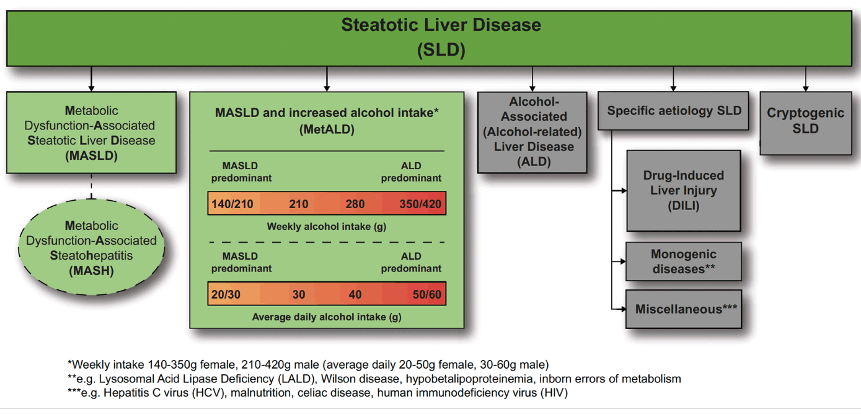
Conclusion: The new nomenclature and diagnostic criteria for steatotic liver disease are widely supported and non-stigmatising, and can improve awareness and patient identification.
Non-alcoholic fatty liver disease (NAFLD) may be more common among people with HIV (PWH), but unique risk factors for NAFLD in PWH (NAFLD-PWH) are poorly understood. In the oral abstract session, a study was presented which examined the prevalence of and risk factors for NAFLD and advanced fibrosis (AF) in a cohort of PWH without other known causes of liver disease, as well as histological features of NAFLD in persons with and without HIV (1). Within this ongoing prospective study, PWH ≥18 years of age on suppressive antiretroviral therapy (ART) were screened for NAFLD (controlled attenuation parameter ≥263 dB/m) and AF (liver stiffness measurement ≥11 kPa) using vibration controlled transient elastography. For histology, 107 biopsies each from NAFLD-PWH (cases) and NAFLD in people without HIV (controls) were matched on age/sex/race/ethnicity/BMI/ALT. Biopsies were centrally read using the NASH Clinical Research Network (CRN) scoring system.
Out of 654 enrolled subjects 53% had a CAP dB/m of >263 dB/m and were thereby classified as having NAFLD. 15% had a LSM ≥ 8 kPa suggestive of relevant fibrosis. Risk factors for NAFLD and advanced fibrosis within the multivariate analysis are shown in figure 21. Older age, male sex, greater BMI or waist circumceference and higher ALT and triglyceride concentrations were associated with great NAFLD odds, and non-Hispanic Black race with lower odds (p<0.05). Greater BMI or waist circumference, higher AST and alkaline phosphatase concentrations and lower platelet counts were associated with greater odds of advanced fibrosis, and non-Hispanic Black race with lower odds (p<0.05).
Figure 21: Risk factors associated with NAFLD and advanced fibrosis.
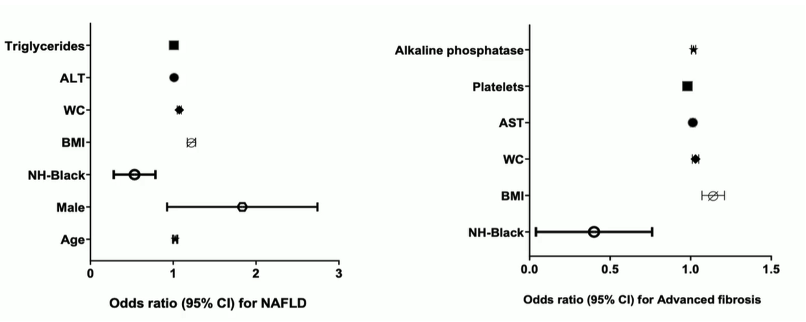
Traditional histologic drivers of fibrosis were less pronounced in NAFLD-PWH and yet fibrosis stage was higher vs. matched controls without HIV, suggesting HIV-specific factors beyond hepatic necroinflammation may contribute to fibrosis in NAFLD-PWH. Indeed, infection of hepatocytes by HIV has been demonstrated which may contribute to more fibrosis progression.
Conclusion: In this cohort of PWH undergoing systematic screening, NAFLD prevalence was high, with traditional metabolic risk factors but not HIV-/ART-specific characteristics dominating risk for NAFLD and AF. This information is important as it does not suggest an own role of ARVs in causing MASLD, which has been discussed controversially for quite some time now.
Metabolic-Associated Steatotic Liver Disease (MASLD) is common among people with HIV (PWH) and likely synergistic with HIV-1 to accelerate hepatic injury and organ dysfunction. The glucagon-like peptide-1 receptor agonist semaglutide is associated with cardiometabolic improvements in the general population through its effects on weight reduction and systemic inflammation. In the oral hepatitis session, the results of a phase IIb, single-arm, pilot study of the effects of semaglutide on magnetic resonance imaging-proton density fat fraction (MRI- PDFF)-quantified intrahepatic triglyceride (IHTG) content in PWH and MASLD was presented (2). ACTG A5371 enrolled PWH ≥18 years of age on suppressive antiretroviral therapy (ART) with central adiposity, insulin resistance or prediabetes and SLD (defined as ≥5% IHTG on MRI-PDFF). All participants received semaglutide for 24 weeks (titrated to 1mg sc weekly by week 4). IHTG content was measured by a central, blinded reader. Primary outcome was change in intrahepatic triglyceride content at week 24.
Participants (n=49) had median age 52 years, BMI 35 kg/m2, 39% Hispanic ethnicity and 33% Black/African American race; 43% were cis or trans women and 82% were on integrase strand transfer inhibitor- based ART. Semaglutide was well-tolerated, with only 2 possibly-related Grade 3 (1 nausea, 1 serotonin syndrome) and no Grade 4 adverse events. Overall, clinically significant reductions in IHTG were observed. 58% of participants had a ≥30% relative reduction in IHTG. 29% of participants had complete MASLD resolution (absolute IHTG <5%). A greater reduction in IHTG was observed in women, Hispanic and non-Hispanic White participants and participants with age >60 years. The primary outcome results are shown below in figure 22.
Figure 22: primary outcome: Changes in IHTG.
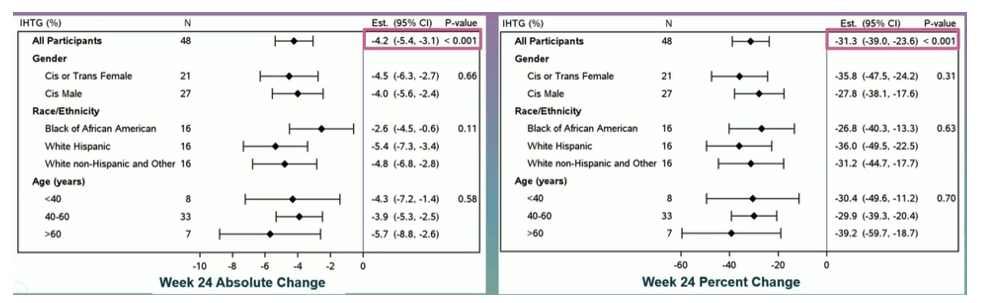
Conclusion: Low-dose (1mg weekly) semaglutide is a safe and effective pharmacologic therapy for MASLD in PWH and shows evidence of broader cardiometabolic benefit. With regard to the lack of treatment options for MASLD currently these data are very encouraging and demand for more clinical trials in this area. Clearly clinical guidance is urgently needed in whom and how to use this new drug class considering the high off-label use of these compounds currently. In particular more data on adverse events and overall safety are needed.
Summary
• In PWH with prior HBV vaccine non-response, both 2 and 3 doses of HepB-CpG achieved superior SPR compared to 3 doses of HepB-alum. The use of the more immunogenic HepB-CpG is already recommended in the EACS guidelines to be considered where available with the aim to reach better seroprotection titers.
• For PWH who fail to achieve high-titer seroresponse after HBV revaccination, more frequent follow-up of anti-HBs titers is recommended to detect the loss of seroprotection for HBV revaccination in a timely manner and allow for revaccination.
• HBV reactivation after switching to anti-HBVsr is unlikely in PLWH with both anti-HBc and anti-HBsAb. Close monitoring of ALT and possibly HBV-DNA is strongly recommended in PLWH with isolated anti-HBc switching to an anti-HBV sparing regimen.
• HDV replication pathway acts independently from the size of intrahepatic HBV reservoir and is fueled by an abundant production of HBs transcripts, mainly derived from integrated HBV-DNA.
• In the era of tenofovir-containing ART, PWH with chronic HBV infection remain at risk for HDV superinfection, and HDV infection is associated with liver-related death.
• Patients with chronic HDV treated with bulevirtide for 96 weeks show substantially reduced levels of HDV RNA together with liver enzyme normalization in up to 56% of treated individuals. Through 2 years of treatment, bulevirtide was safe and well tolerated.
• Preclinical data for a novel Glecaprevir/Pibrentasvir LAI demonstrate sustained therapeutic concentrations in rats over a period of 13 weeks, exceeding the 8-week human target duration.
• In the ALIVE cohort, HIV and HCV seroincidence decreased over time in PWID corresponding with increased prevention efforts and behavioral changes. HCV seroincidence however, remained high. Intensified efforts are needed to achieve HCV elimination among PWID.
• HCV prevalence among people with HIV in Australia has declined below 1% following rapid DAA scale-up and reinfection incidence has declined subsequently as well.
• As simplified treatment approaches are critical for HCV elimination, a 4-week G/P regimen could be tested in implementation programs aiming to cure early HCV at least in low viremic subjects.
• In Thailand there is a high incidence of HCV reinfection in MSM with a short median time to reinfection of 1.3 years. Post-clearance follow-up with counseling and preventive strategies to reduce ongoing risk behavior are needed to reduce HCV reinfection.
• In the absence of cofactors, patients with a baseline LSM ≤ 13.5 kPa or with an LSM <12 kPa and platelet count >150x10^9/L after therapy may not need ongoing surveillance for portal hypertension, including LSM and endoscopy.
• Exposure to sofosbuvir and velpatasvir via breastmilk is minimal. This is reassuring for infant safety with low potential for the development of HCV resistance with perinatal transmission.
• A screening and linkage-to care program for viral hepatitis in migrants in Italy is associated with high rate of acceptation (98%) and treatment efficacy.
• Nontargeted HCV screening was superior to targeted HCV screening for identifying newly diagnosed HCV in the emergency department. Screening in emergency departments can play an important role on the path to HCV elimination.
• Linkage navigation combined with clinician referral (CR) was superior to CR alone for initiating and completing HCV treatment among ED patients identified with HCV.
• The prevalence of HCV viremia in PWH, overall and across key demographic groups, improved over time in the US during the DAA era but still remains higher than in people without HIV.
• In PWH MASLD prevalence is high, with traditional metabolic risk factors but not HIV-/ART-specific characteristics dominating risk for MASLD and advanced fibrosis.
• Low-dose (1mg weekly) semaglutide is a safe and effective pharmacologic therapy for MASLD in PWH and shows evidence of broader cardiometabolic benefit.
References
1. Lake Jordon E et al. NAFLD and Advanced Fibrosis are Common in Adults with HIV and Associated with Unique Histology. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, abstract 158
2. Lake Jordon E et al. Semaglutide Reduces Metabolic-Associated Steatotic Liver Disease in People With HIV: The SLIM LIVER. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, abstract 159
3. Eshan U. Patel, et al., Trends in HIV and HCV Prevention Efforts and Incidence Among People Who Inject Drugs in Baltimore. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, abstract 160
4. Usman Arshad, et al., Preclinical Pharmacokinetic Assessment of a Hepatitis C Virus Long-Acting Injectable Formulation. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, abstract 161
5. D'Anna S et al., Intrahepatic HDV Activity Is Fueled by Integrated HBV DNA Derived HBs Independently From cccDNA Size. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, abstract 162
6. Marks K et al. HepB-CpG Vaccine Is Superior to HepB-alum in People With HIV and Prior Vaccine Nonresponse: A5379. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, abstract 209
7. Zoulim F. Novel Markers of Hepatitis B: Clinical utility for New Treatment strategies. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, abstract 32
8. Price J. Steatotic Liver Disease in Persons Living With HIV. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, abstract 6
9. Hung CC et al. Loss of Serologic Response in Young People with HIV (PWH) Who Had Undergone HBV Revaccination. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 729
10. Avihingsanon A et al. Factors Associated With HBV Response to B/F/TAF Versus DTG + F/TDF at Week 96 in People With HIV-1 and HBV. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 732
11. Morsica G et al. Hepatitis B Reactivation in PLWH With Anti-Core Antibody After Switch to an Anti-HBV Sparing Regimen. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 737
12. Mezzadri L et al. Transaminase elevations among patients with occult HBV infection on two-drug antiretroviral regimens. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 736
13. Marlowe E et al. Epidemiologic Burden of hepatitis D Virus in the United States. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 724
14. Huang JS et al. Incidence and outcome of HDV infection in people with HIV in the era of tenofovir-containing therapy. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 725
15. Wedemeyer H et al. Efficacy and Safety of Bulevirtide 2 mg or 10 mg for 96 Weeks in Chronic Hepatitis Delta, Including in 2 Patients With HIV/Hepatitis B Virus/Hepatitis Delta Virus. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 735
16. Carson J et al. Elimination of HCV among people with HIV in Australia. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 699
17. Ma J et al. Prevalence and Risk Factors for Hepatitis C Viremia in People with HIV in the US During the DAA Era. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 698
18. Mehta SH et al. A Precision Randomized Trial of hepatitis C Treatment Adherence Support among 3000 PWID in India. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 697
19. Kim A et al. A Phase II Trial of 4 Weeks of Glecaprevir/Pibrentasvir for Early Hepatitis C Virus (ACTG A5380). 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 696
20. Promsena P et al. Hepatitis C Reinfection Among Men Who Have Sex with Men in an Acute HIV Cohort in Thailand. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 721
21. Han WM et al. Re-infections following a Minimal Monitoring Approach for Treatment of Hepatitis C Virus Infection. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 722
22. Berenguer J et al. Predictors of liver-related events following DAA HCV cure in PWH with advanced fibrosis/cirrhosis. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 717
23. Chappell CA et al. Transfer of Sofosbuvir/Velpatasvir into Breastmilk. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 711
24. Haukoos J et al. Hepatitis C Screening in the Emergency Department. The Multi-Center DETECT Hep C Clinical Trial. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 707
25. Rowan SE et al. Navigation is Superior to Clinician Referral for Linkage to HCV Care from the Emergency Department. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 708
26. Russo A et al. A Model to Eliminate Viral Hepatitis Infection in Migrants: A Prospective Study in Southern Italy. 31st Conference on Retroviruses and Opportunistic Infections, Denver, March 03-06, 2024, poster 706
27. Marks KM, Kang M, Umbleja T, et al. Immunogenicity and safety of hepatitis B virus (HBV) vaccine with a toll-like receptor 9 agonist adjuvant in HBV vaccine-naive people with human immunodeficiency virus. Clin Infect Dis. 2023;77:414-418.
28. Avihingsanon A, et al. ALLIANCE Study Team. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 and hepatitis B coinfection (ALLIANCE): a double-blind, multicentre, randomised controlled, phase 3 non-inferiority trial. Lancet HIV. 2023 Oct;10(10):e640-e652.
29. Lewis KC, Barker LK, Jiles RB, Gupta N. Estimated Prevalence and Awareness of Hepatitis C Virus Infection Among US Adults: National Health and Nutrition Examination Survey, January 2017-March 2020. Clin Infect Dis. 2023;77(10):1413-1415
30. Solomon SS, et al. A minimal monitoring approach for the treatment of hepatitis C virus infection (ACTG A5360 [MINMON]): a phase 4, open-label, single-arm trial. Lancet Gastroenterol Hepatol. 2022 Apr;7(4):307-317.
31. Rinella ME, et al. NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023 Dec 1;78(6):1966-1986.
|
| |
|
 |
 |
|
|