 |
 |
 |
| |
Biomarker Signatures in Phase Ib Study With PD-1
Inhibitor, Budigalimab, in PLWH Undergoing ATI
|
| |
| |
CROI 2024 March 3-6 Denver
Preethi Krishnan, Ana Gabriela Pires Dos Santos, Harsh Sharthiya, Rakesh L. Tripathi, Thomas J. Reisch, Tanaya Vaidya, Fei Zhou, Moti Ramgopal, Meri Oliva, Aparna Vasanthakumar, Daniel E. Cohen, Jean-Pierre Routy, Patrick K. Dorr
Program Abstract
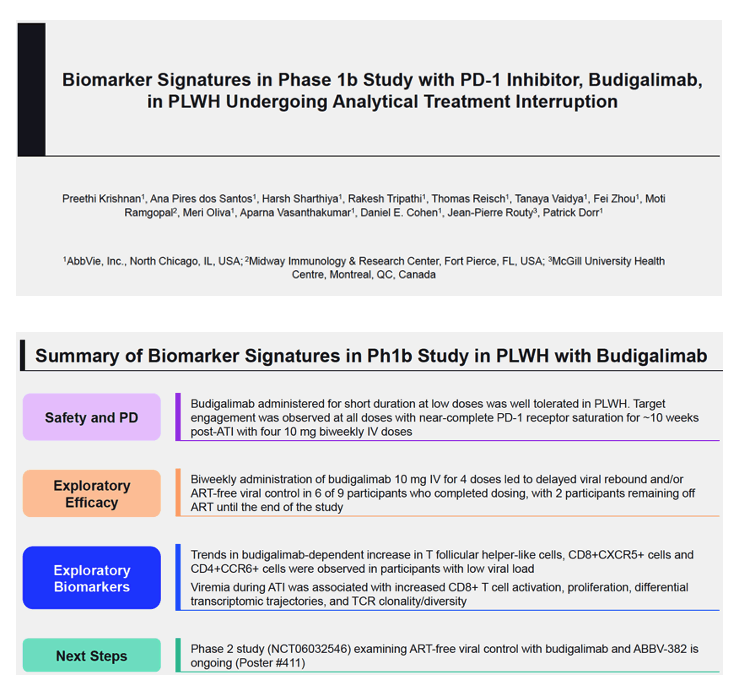
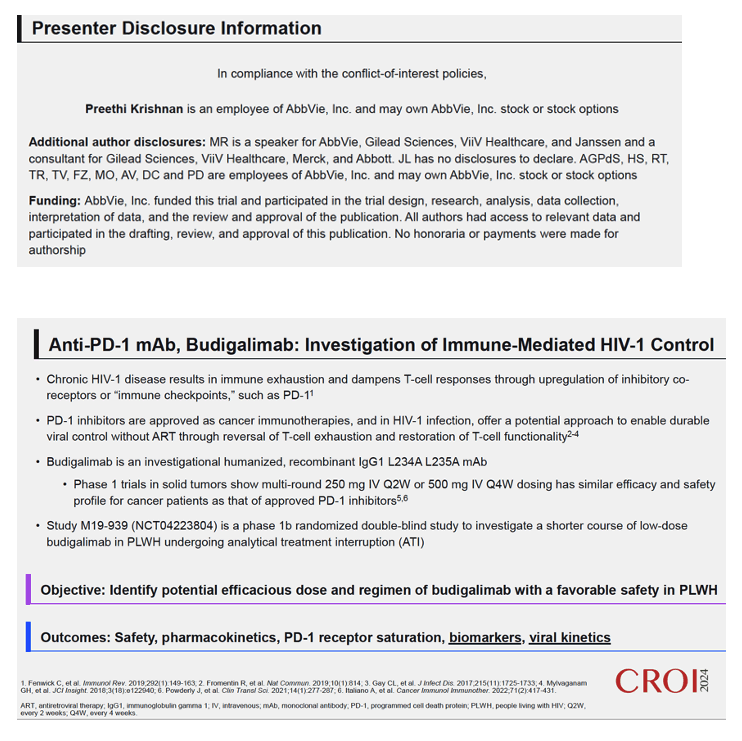
Background: HIV infection is a major global health problem with ART-free viral control remaining an unmet need. PD-1 blockade offers a promising approach to help address this. We conducted a phase 1b randomized double-blind study (NCT04223804) with an investigational PD-1 inhibitor, budigalimab, in people living with HIV-1 (PLWH) and included planned analytical treatment interruption (ATI) enabling exploratory efficacy and biomarker analyses. Budigalimab was well tolerated, and ART-free viral suppression was observed in a subset of participants. Preliminary exploratory biomarker analyses characterizing the impact of treatment and ATI are presented.
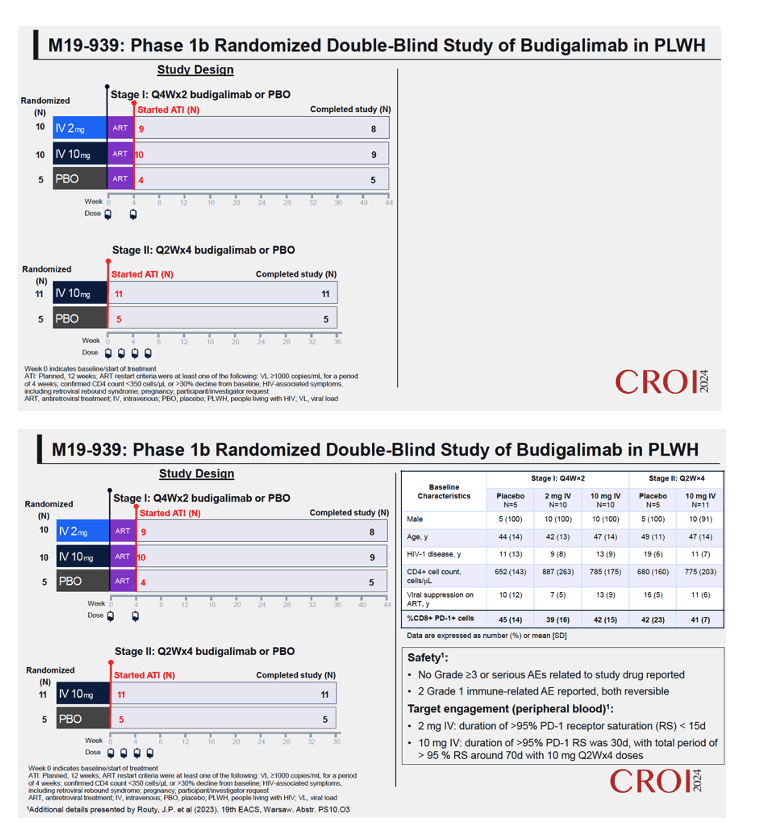
Methods: Safety, pharmacokinetics and pharmacodynamics were examined across multiple intravenous doses of budigalimab (2−10 mg; n=31) and placebo (n=10). Exploratory analyses examined off-ART viral load kinetics, PD-1 receptor saturation, T cell activation and proliferation, plasma cytokines and chemokines, and genome-wide transcriptomic abundances.
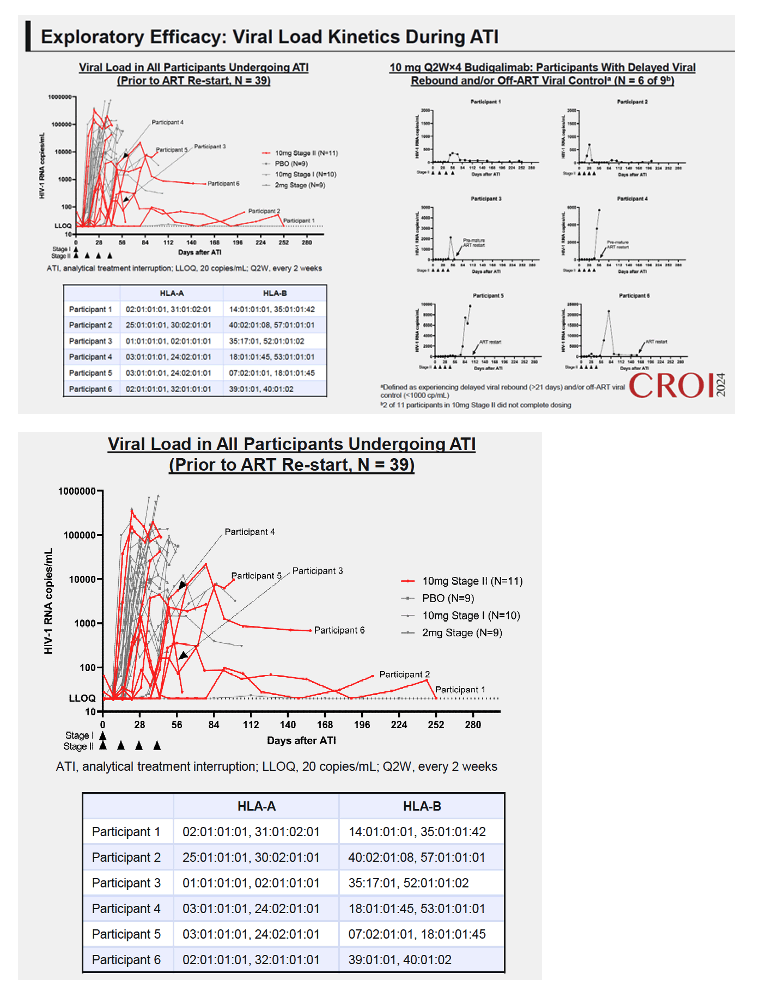
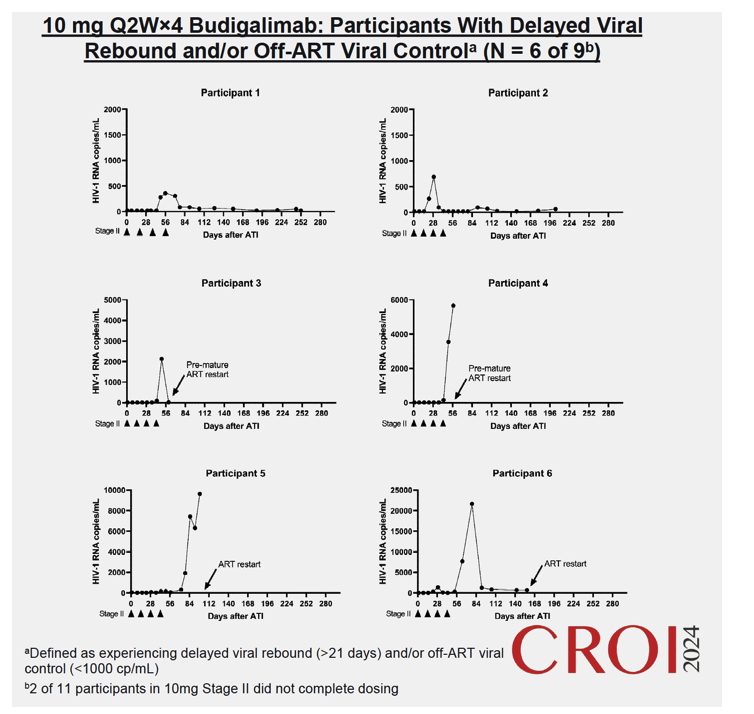
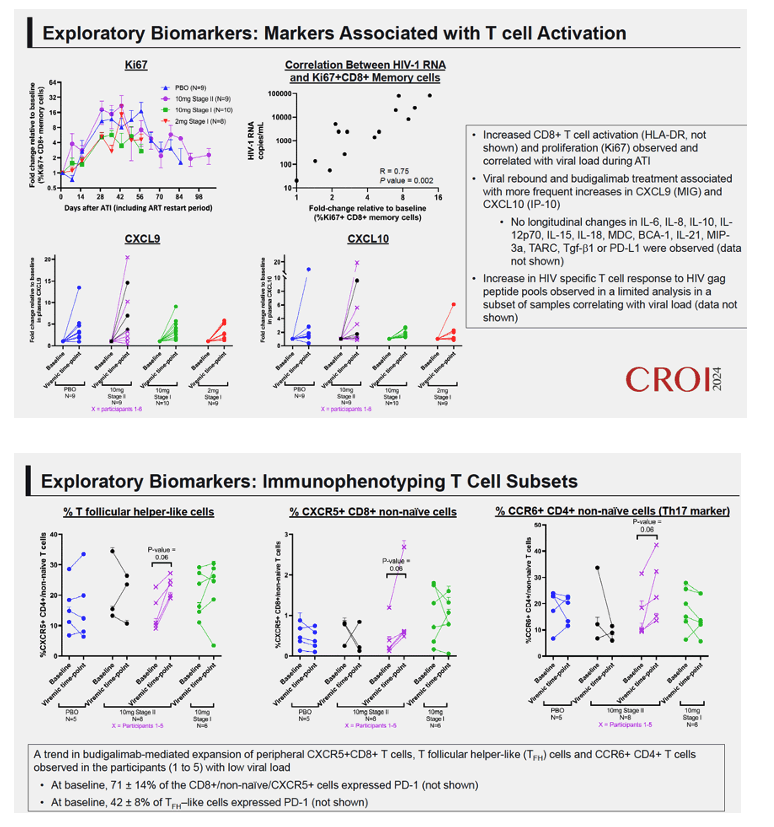
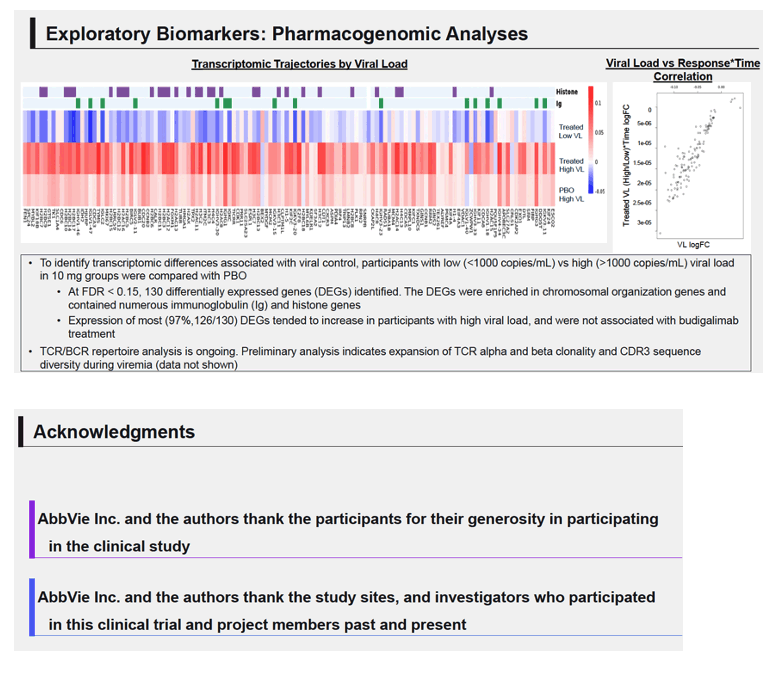
Results: High peripheral PD-1 receptor saturation was observed for a period of approximately 10 weeks with 10mg Q2Wx4 doses of budigalimab. Six of 9 participants who completed the 10mg Q2Wx4 doses administered during ATI had delayed viral rebound and/or off-ART viral control (HIV-1 RNA <1000 copies/mL), with 2 participants maintaining viral control off ART at <200 copies/mL for over 29 weeks. None of the participants receiving placebo demonstrated this viral load kinetic profile. Increased CD8+ T cell activation was observed and correlated with viral load during ATI. Participants with high viral load during ATI displayed differential transcriptomic trajectories as compared to the profile in participants with low viral load (HIV-1 RNA <1000 copies/mL). Budigalimab treatment was associated with more frequent increases in plasma CXCL9 and CXCL10 during ATI as compared to placebo, though there was no association of these markers with treatment response. A trend in budigalimab-mediated expansion of peripheral CXCR5+CD8+ T cells, T follicular helper- like cells and CCR6+CD4+ T cells was observed in the participants with low viral load, potentially contributing to HIV-specific T cell responses.
Conclusion: Low dose budigalimab was well-tolerated in Phase 1b studies in PLWH. The exploratory efficacy and biomarker responses highlighted encouraging characteristics of budigalimab in facilitating immune- mediated viral control, warranting further evaluation in Phase 2 studies.between host immune responses and viral persistence.
|
| |
|
 |
 |
|
|