 |
 |
 |
| |
Long-Acting Injectable CAB/RPV is Superior to
Oral ART in PWH With Adherence Challenges: ACTG A5359
|
| |
| |
CROI 2024 March 3-6 Denver
Written by Jules Levin
On February 12, 2024, a planned interim review by an independent Data and Safety Monitoring Board (DSMB) recommended that the study stop randomizing participants and offer the long-acting injectable treatment to all eligible participants.
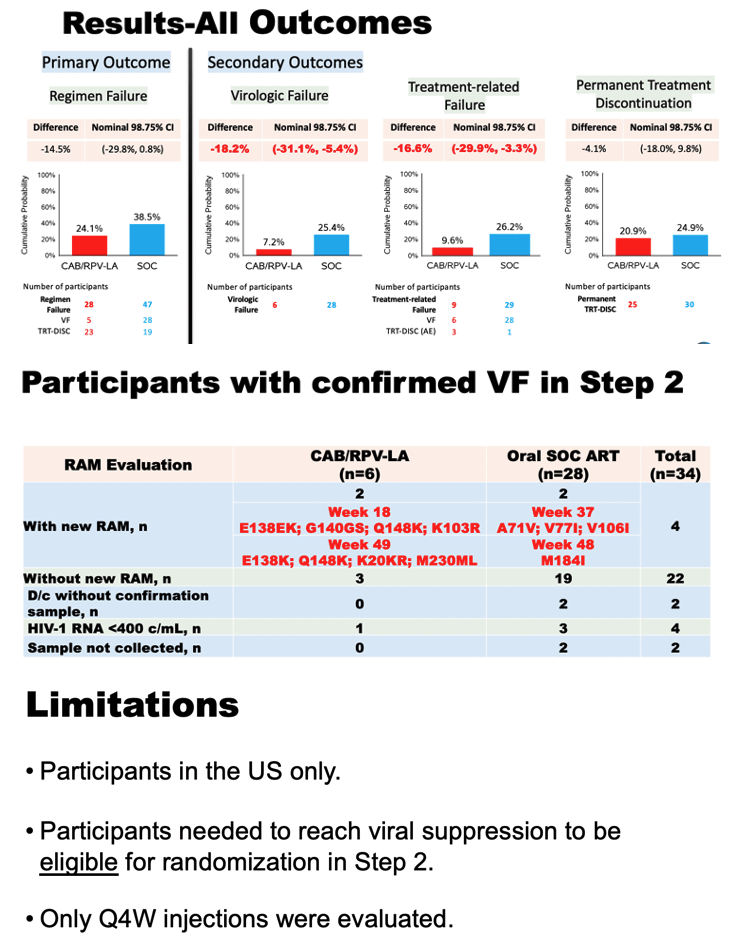
The long-acting injectable treatment was more effective than the daily oral medication at maintaining virologic suppression and avoiding treatment-related discontinuation. Twenty-eight participants receiving the long-acting injectable treatment experienced regimen failure (defined as virologic failure or treatment discontinuation), compared to 47 receiving daily oral medication. The DSMB concluded that there was strong evidence for superiority of the long-acting injectable treatment compared to the daily oral medication in this population, and therefore recommended stopping the randomized portion of the trial. The final analysis of the study efficacy results is ongoing.
"Until now, the population that was able to benefit from the availability of long-acting injectables has been limited to those with durable viral suppression on oral antiretrovirals and no history of failure on oral therapy," said Protocol Chair Aadia Rana, M.D., University of Alabama at Birmingham. "The findings we shared today are an important step toward ultimately ensuring that people living with HIV facing challenges with adherence have access to a treatment option that meets their health needs, allowing them the potential to experience the health benefits of becoming virally suppressed."
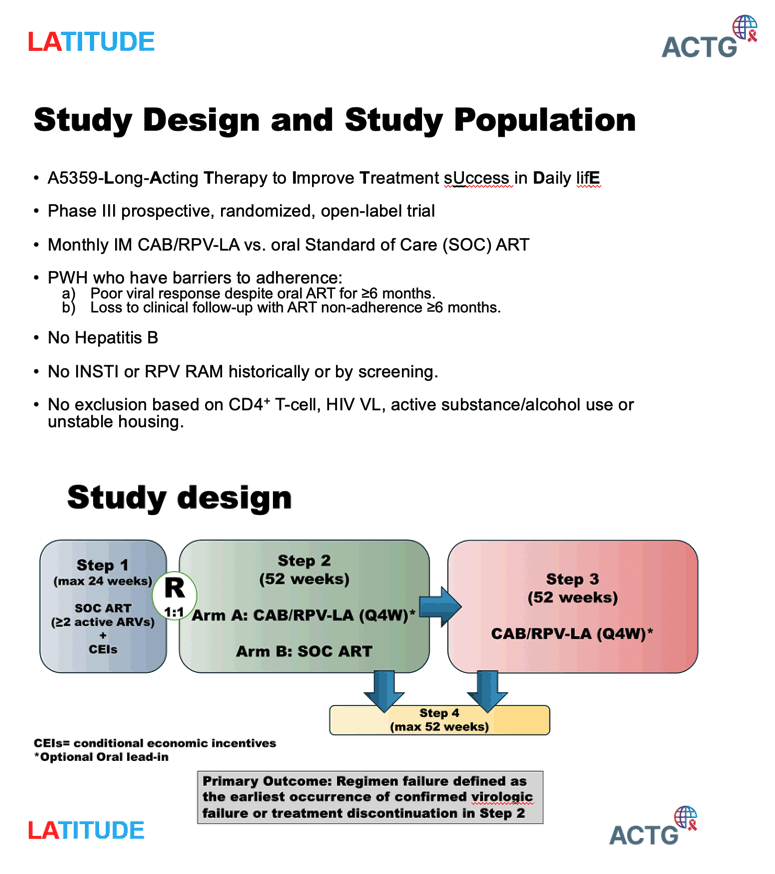
The interim data upon which the DSMB recommendations were made include 434 participants who were enrolled in the first step of the study (when all participants receive daily oral medication). The median age was 40 years old; 40 percent of participants were male, 64 percent were Black/African American, 17 percent were Hispanic, 5 percent were transgender, and 14 percent currently or previously used injection drugs. They had a median of 270/mm3 CD4+ cells and a median of 3.55 log10 c/mL HIV-1 RNA. Of the 294 eligible participants who were randomized in step 2 of the study, 146 received the long-acting injectable treatment and 148 continued to receive daily oral medication.


|
| |
|
 |
 |
|
|