 |
 |
 |
| |
A DOSE ESCALATION STUDY OF SAFETY & PK OF
TMB-365 & TMB-380 IN PEOPLE WITH SUPPRESSED HIV
|
| |
| |
CROI 2024 March 3-6 Denver
Jacob P. Lalezari1, Moti Ramgopal2, Gary Richmond3 , Gordon Crofoot4, Kuei-Ling Kuo5, YingAn Lai5,Martin Markowitz6
1Quest Clinical Research, San Francisco, CA, USA, 2Midway Immunology and Research Center, Fort Pierce, FL, USA, 3CAN Community Health, Fort Lauderdale, FL, USA, 4Crofoot Research Center, Houston, TX, USA, 5TaiMed Biologics Inc., Taipei City, Taiwan, 6TaiMed Biologics Inc., Irvine, CA, USA
Abstract
TMB-365, a second generation post-attachment bNAb, binds to the second domain of CD4 and is designed to display improved PK, antiviral activity, and breadth of coverage when compared to ibalizumab. TMB-380 (aka VRC07-523LS) is a bNAb that binds to the CD4 binding site of HIV Env and has potent antiviral activity and favorable safety and PK profile. The combination is designed as a complete regimen for HIV therapy. Here we present available safety and PK results of the combination given as a single IV infusion to inform the feasibility of maintenance therapy given every 8-12 weeks in suppressed HIV-infected individuals.
Three groups of 10 subjects were infused with 2400 mg (Group 1), 3200 mg (Group 2), or 4800 mg (Group 3) of each bNAb in 250 ml NS over one hour and followed for 12 to 16 weeks. Groups were dosed sequentially with dose escalation approved by an independent Data Monitoring Committee. Participants were suppressed with continuous oral cART for at least 6 months, clinically stable, without a history of severe allergies, and had no history of virologic failure on previous treatment regimens. All infusions were performed over 60 minutes in outpatient clinics with vital sign determinations every 15 minutes during and up to 3-5 hours post-infusion. A subset of participants was pre-medicated with acetaminophen 650 mg and cetirizine 10 mg at the investigator's discretion. Trough PK targets were ≥0.3 μg/ml and 65 μg/ml for TMB-365 and TMB-380, respectively in at least 80% of participants in any treatment group.
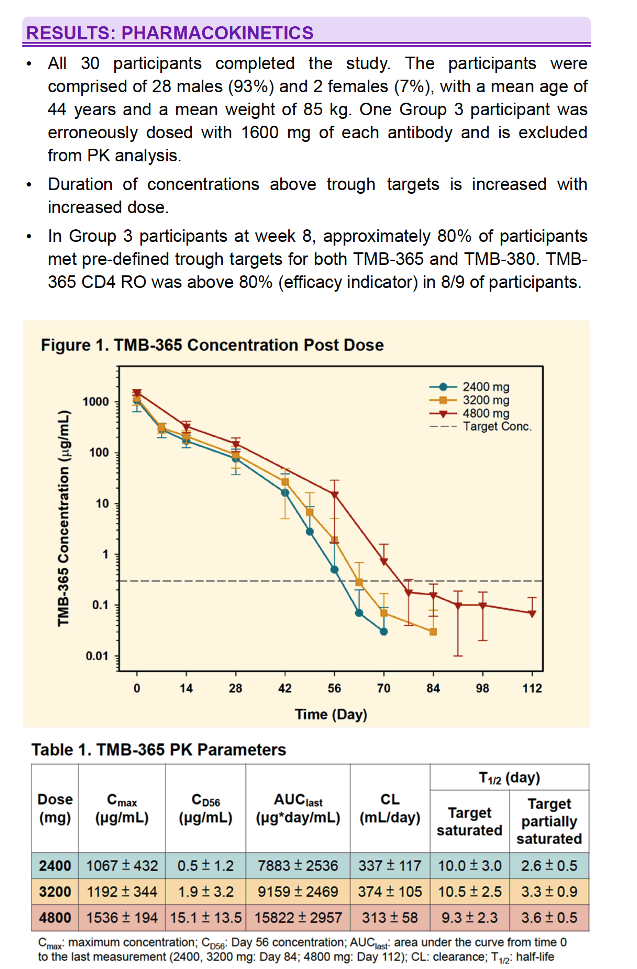
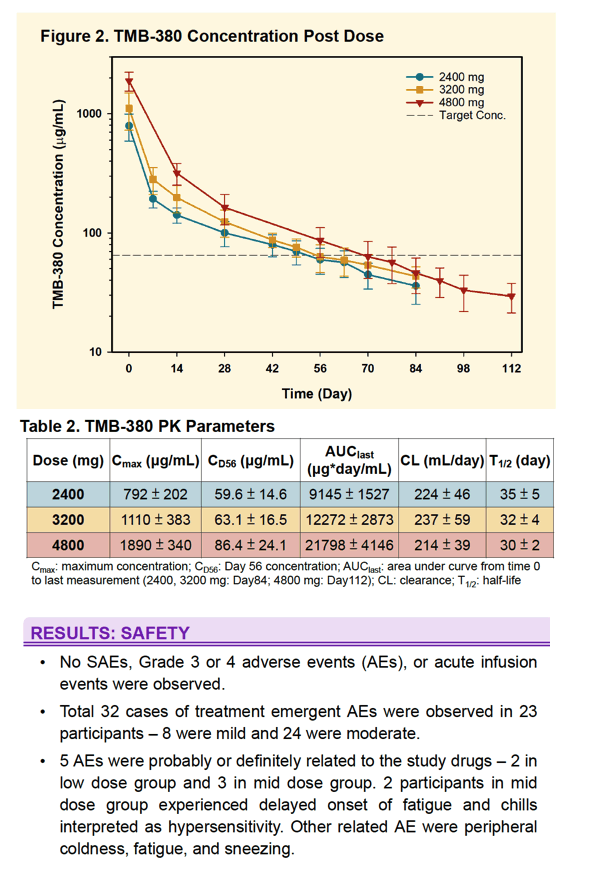
All 30 subjects completed the study. One Group 3 subject was erroneously dosed with 1600 mg of each antibody and is excluded from PK analysis. No SAEs, Grade 3 or 4 adverse events, or acute infusion events were observed. Treatment emergent AEs (N=32) were mild to moderate. 5 were attributed to infusions with bNAbs. 2 subjects experienced delayed onset of fatigue and chills interpreted as hypersensitivity. PK data is shown below. Duration of concentrations above trough targets is increased with increased dose. In Group 3 participants at week 8, mean TMB-365 and TMB-380 concentrations were 15.1 and 86.4 μg/ml, respectively, and approximately 80% of participants met pre-defined trough targets.
A single infusion of TMB-365 and TMB-380 in combination up to 4800 mg each is safe. Prolonged PK duration was observed for both TMB-365 and TMB-380 and results suggest that an every 8-week infusion is feasible and will be tested in a Phase 2 clinical study.

|
| |
|
 |
 |
|
|