| |
Efficacy, safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir for HIV pre-exposure prophylaxis in transgender women: a secondary analysis of the HPTN 083 trial
|
| |
| |
Sept 2023
Results from this study specific to transgender women are consistent with the overall HPTN 083 study result, which found that injectable cabotegravir administered intramuscularly every 8 weeks was well tolerated, safe, and highly effective in preventing HIV infection in men who have sex with men and transgender women at substantial risk for acquiring HIV.13
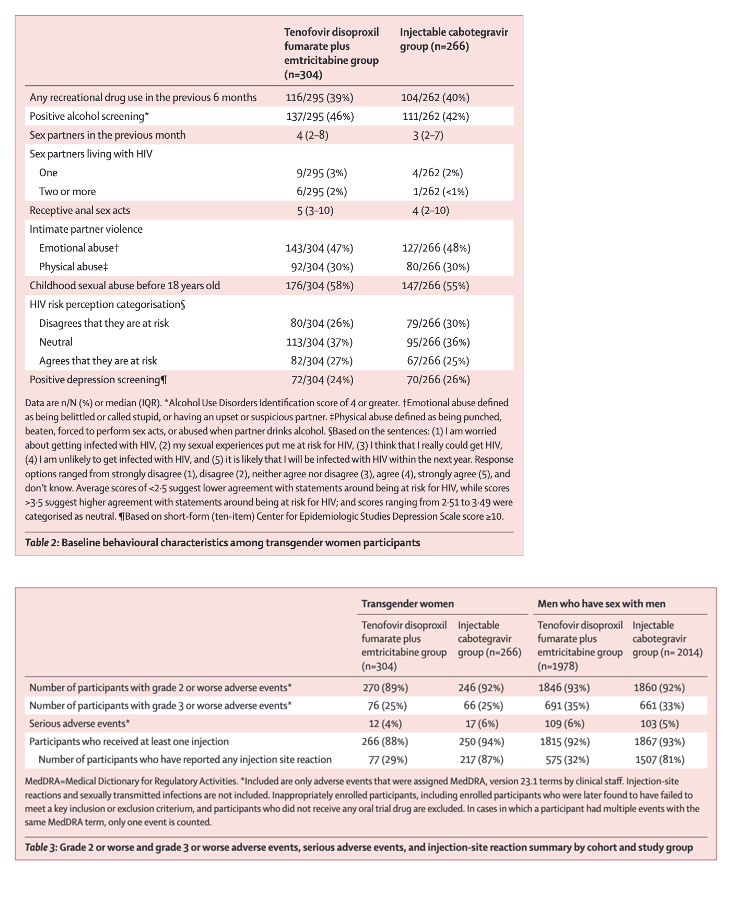
Cabotegravir concentrations were not statistically different between transgender women who reported using gender affirming hormone therapy compared with transgender women who reported not using this therapy. However, additional studies, which capture detailed gender affirming hormone therapy dosing information and hormone concentrations, are needed to more fully characterise the relationship between cabotegravir and these therapies.
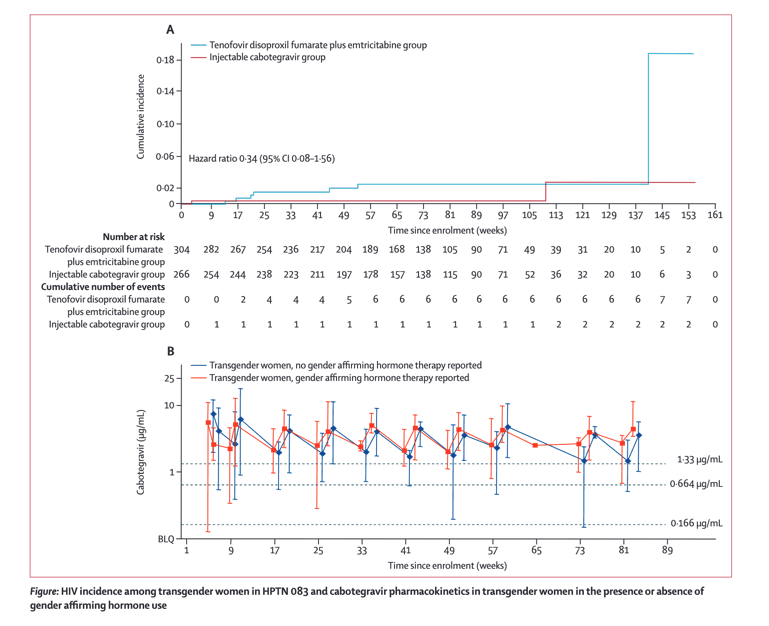
Nine seroconversions occurred among transgender women during the blinded phase of the study (seven in the tenofovir disoproxil fumarate plus emtricitabine group and two in the injectable cabotegravir group); overall incidence was 1⋅19 per 100 person-years (95% CI 0⋅54-2⋅25): 1⋅80 per 100 person-years (0⋅73-3⋅72) in the tenofovir disoproxil fumarate plus emtricitabine group and 0⋅54 per 100 person-years (0⋅07-1⋅95) in the injectable cabotegravir group (hazard ratio 0⋅34 [95% CI 0⋅08-1⋅56]). Cabotegravir concentrations did not differ by gender affirming hormone therapy use.
Interpretation
HIV prevention strategies for transgender women cannot be addressed separately from social and structural vulnerabilities. Transgender women were well represented in HPTN 083 and should continue to be prioritised in HIV prevention studies. Our results suggest that injectable cabotegravir is a safe and effective pre-exposure prophylaxis option for transgender women.
Summary
Background
The HIV Prevention Trials Network (HPTN) 083 trial showed that long-acting injectable cabotegravir was more effective than tenofovir disoproxil fumarate plus emtricitabine in preventing HIV in cisgender men and transgender women who have sex with men. We aimed to characterise the cohort of transgender women included in HPTN 083.
Methods
HPTN 083 is an ongoing, phase 2b/3, randomised, multicentre, double-blind, double-dummy clinical trial done at 43 sites in seven countries (Argentina, Brazil, Peru, the USA, South Africa, Thailand, and Viet Nam). HIV-negative participants were randomly assigned (1:1) to receive injectable cabotegravir or tenofovir disoproxil fumarate plus emtricitabine. The study design and primary outcomes of the blinded phase of HPTN 083 have already been reported. An enrolment minimum of 10% transgender women was set for the trial. Here we characterise the cohort of transgender women enrolled from Dec 6, 2016, to May 14, 2020, when the study was unblinded. We report sociodemographic characteristics, use of gender affirming hormone therapy, and behavioural assessments of the transgender women participants. Laboratory testing and safety evaluations are also reported. The trial is registered at ClinicalTrials.gov, NCT02720094.
Findings
HPTN 083 enrolled 570 transgender women (304 tenofovir disoproxil fumarate plus emtricitabine; 266 injectable cabotegravir). Transgender women were primarily from Asia (225 [39%]) and Latin America (205 [36%]); 330 (58%) reported using gender affirming hormone therapy. Intimate partner violence was common (270 [47%] reported emotional abuse and 172 [30%] reported physical abuse) and 323 (57%) reported a history of childhood sexual abuse.
159 (28%) transgender women disagreed that they were at risk for HIV, and 142 (25%) screened positive for depressive symptoms. During study follow-up, incidence of syphilis was 16⋅25% (95% CI 13⋅28-19⋅69), rectal gonorrhoea was 11⋅66% (9⋅14-14⋅66), and chlamydia was 20⋅61% (17⋅20-24⋅49). Frequency of adverse events was similar between the treatment groups.
Nine seroconversions occurred among transgender women during the blinded phase of the study (seven in the tenofovir disoproxil fumarate plus emtricitabine group and two in the injectable cabotegravir group); overall incidence was 1⋅19 per 100 person-years (95% CI 0⋅54-2⋅25): 1⋅80 per 100 person-years (0⋅73-3⋅72) in the tenofovir disoproxil fumarate plus emtricitabine group and 0⋅54 per 100 person-years (0⋅07-1⋅95) in the injectable cabotegravir group (hazard ratio 0⋅34 [95% CI 0⋅08-1⋅56]). Cabotegravir concentrations did not differ by gender affirming hormone therapy use.
Interpretation
HIV prevention strategies for transgender women cannot be addressed separately from social and structural vulnerabilities. Transgender women were well represented in HPTN 083 and should continue to be prioritised in HIV prevention studies. Our results suggest that injectable cabotegravir is a safe and effective pre-exposure prophylaxis option for transgender women.
Funding
National Institute of Allergy and Infectious Diseases and ViiV Healthcare.
|
|
| |
| |
|
|
|