 |
 |
 |
| |
A pilot, randomized controlled trial of doxycycline pre-exposure prophylaxis versus placebo for prevention of bacterial sexually transmitted infections in men who have sex with men living with HIV
|
| |
| |
AIDS 2024 July 20-26 Munich
Troy Grennan, BC Centre for Disease Control, Vancouver, Canada, et al.
abstract
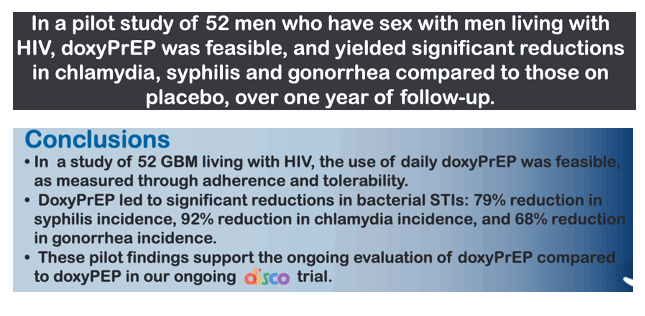
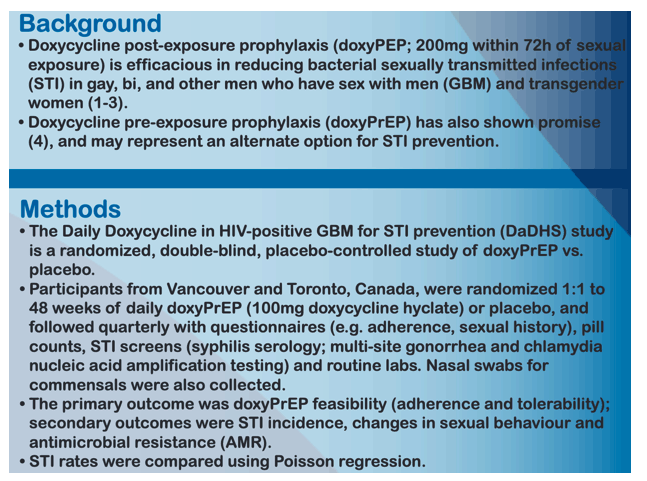
BACKGROUND: Doxycycline post-exposure prophylaxis (doxyPEP) is efficacious in preventing bacterial sexually transmitted infections (STI) in men who have sex with men (MSM). Doxycycline pre-exposure prophylaxis (doxyPrEP) has also shown promise and may represent another option for STI prevention. We undertook a pilot study of doxyPrEP in MSM living with HIV.
METHODS: MSM living with HIV with previous syphilis were randomized 1:1 to receive 48 weeks of daily doxycycline 100mg orally versus placebo in this double-blind pilot study in Toronto and Vancouver, Canada. Participants were followed quarterly with STI screens (syphilis serology, gonorrhea/chlamydia nucleic acid amplification testing of urine, pharynx, and rectum), adherence and sexual behaviour questionnaires, and adverse event (AE) assessments. Nasal swabs were collected to evaluate the emergence of doxycycline resistance in Staphylococcus aureus carriers. STI rates were compared between arms using Poisson regression.
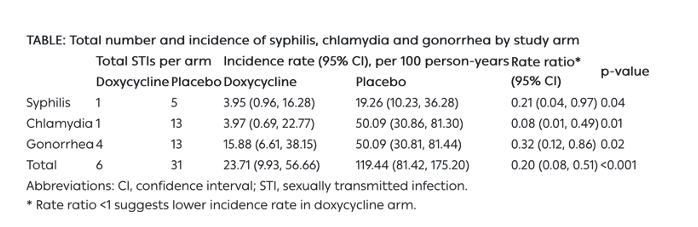
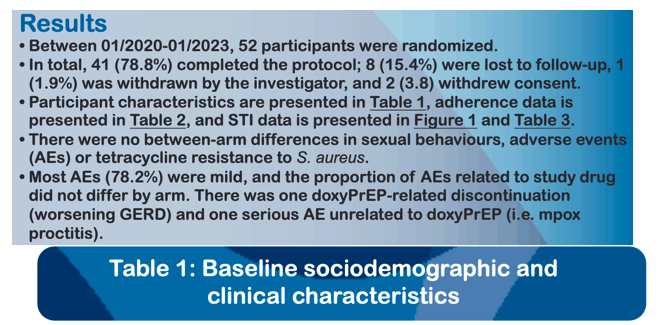
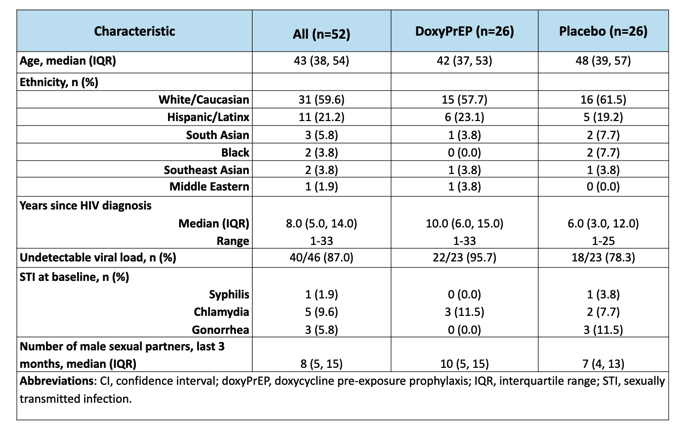
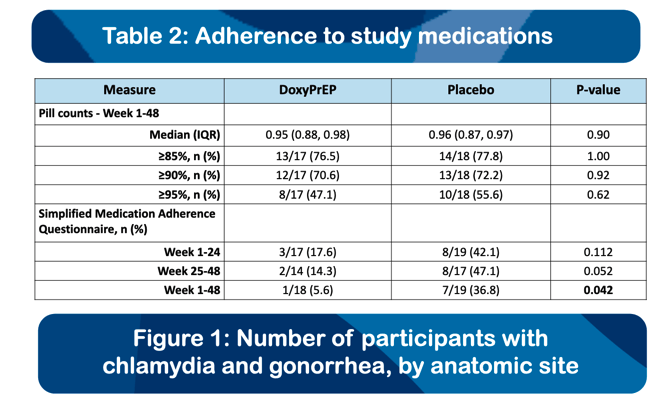
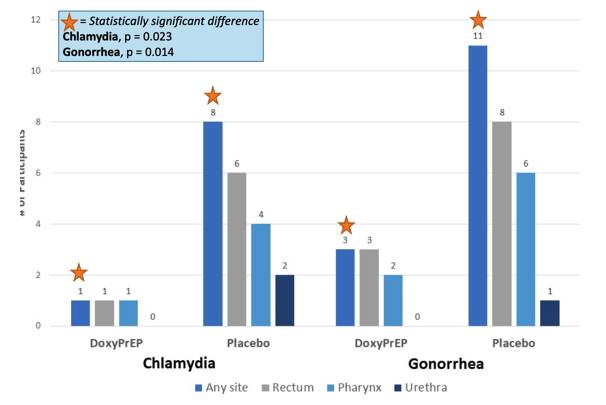
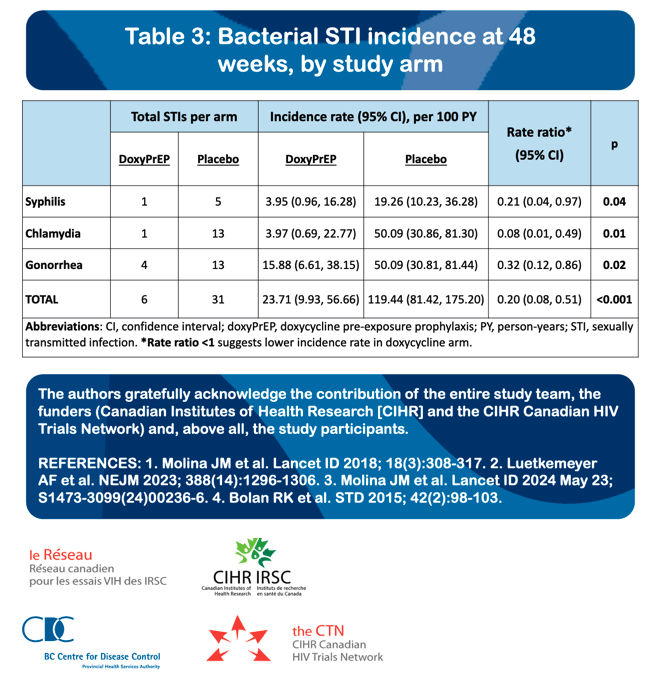
Fifty-two MSM were enrolled with median age of 43 years (interquartile range [IQR], 38-54), from 01/2020 to 01/2023. Forty-one participants (78.8%) completed the study protocol. We observed a reduction of 79%, 92%, and 68% in syphilis, chlamydia, and gonorrhea, respectively, in the doxycycline arm relative to placebo (Table). There were no between-arm differences in drug adherence (pill count) or sexual behaviours (number of partners, condomless sex acts) at any time point. Most AEs (78.4%) were mild, and the proportion of AEs related to study drug did not differ by study arm.. There was one drug-related discontinuation (worsening gastroesophageal reflux), and one serious adverse event (mpox-related proctitis; unexpected and unrelated to drug), both in the doxycycline arm. New doxycycline resistance developed in 3/19 and 2/19 (p=0.57) S. aureus isolates from baseline to week 48 in the doxycycline and placebo arms, respectively.
CONCLUSIONS: DoxyPrEP significantly decreased rates of syphilis, chlamydia and gonorrhea compared to placebo, and was well-tolerated in MSM living with HIV. These pilot findings support our further evaluation of doxyPrEP compared to doxyPEP in an ongoing larger trial.
|
| |
|
 |
 |
|
|