 |
 |
 |
| |
HIV Prevention at CROI 2013
|
| |
| |
20th Conference on Retroviruses and Opportunistic Infections
Seattle, WA
March 3-6, 2011
Jared Baeten, MD PhD
Connie Celum, MD MPH
University of Washington
CROI 2013 - the 20th CROI - was a dynamic, international meeting that addressed all areas of HIV research: pathogenesis, treatment, cure, associated infections, pediatric and adult disease, and prevention. As in previous years, oral sessions were recorded and are available online (www.retroconference.org), along with full copies of abstracts, including abstracts from the prior years of CROI. We have cited specific links here for certain oral sessions - these contain both slides and video, but alternative links with slides with audio only are available.
The Monday morning plenary session included Francois Dabis presenting a talk entitled "Reality check: is the end of AIDS in sight?" The past year has seen tremendous enthusiasm, in many ways rightly-placed but sometimes hyperbole, regarding whether exciting new HIV prevention strategies, most prominently antiretroviral treatment but also advances in pre-exposure prophylaxis (PrEP), microbicides, HIV testing, and in implementation of male circumcision and behavior change, might signal a radical change in the global epidemic. This plenary (http://webcasts.retroconference.org/console/player/19407?mediaType=podiumVideo) provided a strong perspective on the balance to be struck between optimism, reality, remaining questions, and new work to be done.
The first oral abstract sessions of the conference featured an entire morning on prevention:
http://webcasts.retroconference.org/console/player/19409?mediaType=podiumVideo. The topics included PrEP, microbicides, testing, and risk factors for HIV in highest-risk settings. It was a powerful and challenging session.
Pre-exposure prophylaxis (PrEP) for HIV prevention
A major focus of the last three meetings of CROI has been sorting out the findings of different randomized clinical trials of PrEP for HIV prevention. In PrEP, an HIV-uninfected person uses an antiretroviral medication - to date, both single agent and dual-agent PrEP medications have been tested, as oral pills or topical microbicides - ahead of an HIV exposure in order to prevent infection. Multiple trials of PrEP were initiated in the mid-/late 2000s and a steady stream of sometimes-conflicting results has been a challenge for the field.
The first PrEP trial to report results was CAPRISA 004 (reported at IAS 2010), which tested peri-coitally applied 1% tenofovir vaginal gel among 889 South African women and found that tenofovir gel used in this fashion decreased HIV risk by 39% (p=0.017) (Abdool Karim et al., Science 2010). In late 2010, iPrEx, which evaluated daily oral emtricitabine/tenofovir (FTC/TDF) among 2499 men who have sex with men and transgender women from six countries, report that oral PrEP reduced HIV risk by 44% (p=0.005) (Grant et al., New England Journal of Medicine 2010). In both trials, protection against HIV was substantially higher among those with high adherence. In 2011, three PrEP trials released results and findings were published in 2012. The Partners PrEP Study, which followed 4747 HIV serodiscordant couples from Uganda and Kenya and evaluated oral TDF and oral FTC/TDF as PrEP demonstrated that PrEP significantly reduced HIV incidence heterosexual women and men who were at high risk of infection because of having a known HIV-infected partner (TDF 67%, p<0.001 and FTC/TDF 75%, p<0.001; Baeten et al., New England Journal of Medicine 2012). The TDF2 study, which tested daily oral FTC/TDF in a study of 1200 young heterosexuals from Botswana, also demonstrated high efficacy for PrEP (63%, p=0.01; Thigpen et al., New England Journal of Medicine 2012). In both Partners PrEP and TDF2, like in iPrEx, adherence to PrEP appeared to be very strongly related to its HIV protection benefits. Indeed, iPrEx and Partners PrEP, those who were taking PrEP (based on detection of the study medication in blood samples), appeared to reduce their risk of HIV by ~90%. On the basis of the positive results from iPrEx, Partners PrEP, and TDF2, the US Food and Drug Administration (FDA) in 2012 approved daily oral FTC/TDF as the first PrEP agent with a formal label indication for prevention of sexual HIV acquisition.
However, not all PrEP trials found such positive results. The FEM-PrEP Study (Van Damme et al., New England Journal of Medicine 2012) failed to show HIV protection using daily oral FTC/TDF among 2120 African women. The principal reason for the failure of efficacy in FEM-PrEP has been determined to be lack of adherence to the study medication - with less than 30% of study participants having detectable tenofovir in blood samples obtained at intervals in the study. At CROI 2013, the results of the VOICE study, the final large trial of daily oral tenofovir-based PrEP for the prevention of sexual HIV transmission, were reported.
VOICE (Marrazzo, abstract 26LB). VOICE was a phase IIB, randomized, double-blind, placebo-controlled, five-arm trial of daily oral TDF and FTC/TDF and daily vaginal tenofovir gel (the same gel as in CAPRISA 004 but dosed daily in VOICE) as PrEP for the prevention of HIV acquisition by HIV seronegative women. 5029 women, who had reported having vaginal sex in the 3 months prior to enrollment and who were willing to use contraception, were enrolled at sites in South Africa (which, at more than 4000 subjects, carried the bulk of participants and HIV seroconversions), Zimbabwe, and Uganda. Like in other PrEP trials, participants were seen monthly, for HIV and pregnancy testing, safety monitoring, and individualized adherence counseling, and all participants received a comprehensive package of HIV prevention services. The trial began in September 2009. At two interim reviews by the study's Data and Safety Monitoring Board (DSMB), major changes in the trial's design were made: in September 2011, the oral TDF arm was stopped because it appeared to not be effective for HIV prevention, and in November 2011, the trial's vaginal tenofovir gel arm was stopped (and its placebo gel arm) for the same reason. Detailed results of HIV efficacy from the TDF and tenofovir gel arms were not made available until CROI 2013, when the full results of the trial, including for the daily oral FTC/TDF arm, were presented.
The mean age of VOICE participants was 25.3 years, and 51% were less than 25 years of age. Only 21% were married, 71% used injectable contraception, 85% reported condom use with the last sex act, and 17% reported anal sex in the 3 months prior to enrollment. Retention at the end of the study was 91%. Adherence to study product was measured in multiple ways while the study was ongoing: monthly count of unused study product (pills/applicators), monthly self-report, and quarterly ACASI - all suggested ~90% adherence.
There were a total of 334 HIV seroconversions in VOICE, of which 22 were subsequently found to be acute, seronegative infections ("window period") at the time of enrollment, resulting in 312 infections being included in the trial's primary modified intention to treat analysis. For all study arms, randomization to PrEP, compared to placebo, was not associated with reduced HIV incidence: oral TDF incidence 6.3 per 100 woman-years vs. oral placebo incidence 4.2 per 100 woman-years; oral FTC/TDF incidence 4.7 per 100 woman-years vs. oral placebo incidence 4.6 per 100 woman-years; tenofovir vaginal gel incidence 5.9 per 100 woman-years vs. gel placebo incidence 6.8 per 100 woman-years. None of these comparisons were statistically significant: oral TDF efficacy -48% (p=0.07), oral FTC/TDF efficacy (-4%, p>0.2), vaginal tenofovir gel efficacy (15%, p=0.2). All products appeared safe - the frequencies of serious adverse events and laboratory adverse events were similar for those assigned PrEP compared to those assigned placebo.
Like prior PrEP trials, VOICE tested blood samples from all HIV seroconverters and a subset of non-seroconverters from the active PrEP arms (oral TDF and FTC/TDF and tenofovir vaginal gel; N=773, covering 3298 samples). Overall, tenofovir was detected in an average of ≤30% of samples, the likelihood of detection decreased over time, and ≥50% of women in each of the active arms never had tenofovir detected, at any time during their follow-up. The principal predictors of non-detection of tenofovir in blood samples were missing the prior visit, being less than age 25 years, and being unmarried; unfortunately, these factors also were associated with increased risk of HIV.
Thus, the VOICE results confirm the findings of FEM-PrEP: in a randomized, blinded, placebo-controlled trial of African women, PrEP adherence was very low and the trial failed to demonstrate HIV protection as a result of PrEP in the study population. In both the FEM-PrEP and VOICE trials, many of the participants were from South Africa, where HIV incidence was >5% per year, an astonishingly high rate.
The results of VOICE complete a long and challenging course for PrEP for HIV prevention. PrEP, particularly oral PrEP for which there are multiple studies demonstrating efficacy, works for preventing HIV, but only if it is taken. Understanding determinants of PrEP taking is a challenge for the field - it is not likely as simple as being young (iPrEx average age was also <25 years), or young and from southern Africa (given the efficacy in TDF2). At CROI 2013, a poster (Murnane, abstract 1000), presented HIV protection efficacy for a number of higher-risk subgroups from the Partners PrEP Study, including groups limited to women, to younger persons, to those whose partners had high plasma HIV viral loads, and to those for whom placebo arm incidence was ≥5 per 100 person-years. In all groups, PrPE efficacy was high - suggesting that, in population in which PrEP adherence can be motivated, PrEP is highly efficacious for HIV prevention.
Nevertheless, the VOICE results demonstrate that the prevention field collectively has much work to do to develop, evaluate, and implement HIV prevention strategies that will have impact for young women at very high risk of HIV. The results of PrEP trials are summarized in the table below.
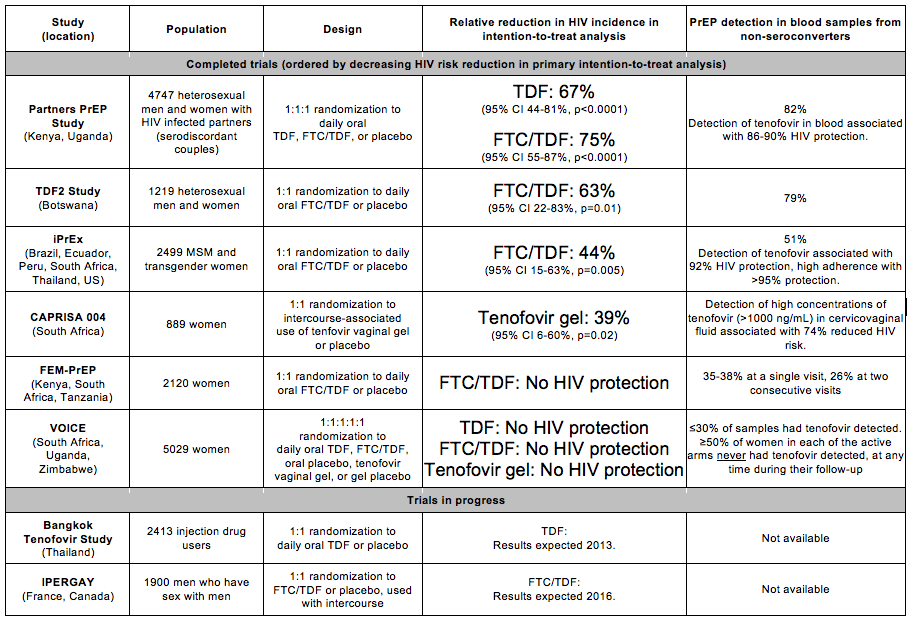
iPrEx (Grant, abstract 27). iPrEx was a phase III trial of FTC/TDF PrEP among men who have sex with men and transgender women. The average age was 24 years, placebo arm HIV incidence was 3.9 per 100 person-years, and PrEP efficacy was 44% in the placebo-controlled comparison but estimated to be >95% for those with high adherence. The iPrEx trial ended in November 2010, efficacy was reported soon afterwards, and active PrEP was offered to study participants through the iPrEx Open Label Extension (OLE) study, which was initiated in June 2011. At CROI 2013, the iPrEx team reported on HIV incidence during the gap period between the end of the trial and opening of iPrEx OLE, examining both the possibility that PrEP delayed ("masked") HIV seroconversion and sexual behavior during the gap. For individual participants, the gap ranged from 7 to 19 months, and participants had access to services at study sites but not PrEP.
About 2/3 of iPrEx participants were interested in continuing in iPrEx OLE, and those who continued were a somewhat behaviorally higher risk population. During the gap, HIV incidence was 4.1 per 100 person-years in those who had been in the trial's placebo arm and 3.3 per 100 person-years in those who had been in the trial's FTC/TDF PrEP arm, demonstrating both 1) no increased HIV risk (suggesting delayed HIV seroconversion) in those who had received PrEP and 2) ongoing HIV risk for study participants after PrEP was discontinued. Risk factors for HIV included lack of condom use, younger age, and herpes simplex virus type 2 (HSV-2) infection. By participant self-report of behavior collected over time, it appeared that participants moved in and out of periods of higher HIV risk. HIV incidence in the now-ongoing iPrEx OLE cohort - with access to open-label PrEP - will be important as well as PrEP adherence, particularly during "seasons" of HIV risk.
CROI: New HIV Rate High After iPrEx Men Stop Truvada PrEP (or Placebo): when taking Truvada PrEP it works
New PrEP agents. Two oral abstracts in this same session described potential new, long-acting PrEP agents, which may avert some of the adherence challenges faced by daily oral/vaginal PrEP.
GSK744 long-acting (Andrews, abstract 24LB). GSK744 is a potent integrase inhibitor being formulated as a long-acting nanosuspension (GSK744LAP) for injectable use as PrEP. In human volunteers, the half-life of GSK744LAP is on the order of ≥3 weeks, supporting the concept of monthly to quarterly injections for regular use. Andrews et al. reported the result of an 8-week macaque model weekly rectal challenge SHIV study using GSK744LAP, given at baseline and week 3. All 8 placebo macaques became infected (after a median of 2 challenges) compared with none of the 8 animals receiving GSK744LAP (p<0.001). Further work on agents like GSK744LAP will be important, as long-acting, user-independent PrEP agents offer opportunities to overcome adherence challenges seen in some of the trials of tenofovir-based daily oral PrEP.
CROI: PrEP GSK744 Integrase Administered Monthly Perhaps Quarterly Prevents HIV-Infection in Monkeys - (03/07/1)
Tenofovir vaginal ring (Smith, abstract 25LB). Intravaginal, slow-release rings containing antiretrovirals for PrEP offer a potentially powerful opportunity to prevent HIV while minimizing systemic medication exposure, and also overcome some challenges to PrEP adherence. A tenofovir intravaginal ring containing 120 mg of TDF, releasing 2.4-2.7 mg/day in vitro and in macaques and resulting in high tissue concentrations of the medication, was developed. In a macaque low-dose vaginal challenge study, 11 of 12 control animals became infected, after a median of 4 exposures. In contrast, full protection was seen in 6 animals receiving the TDF intravaginal ring. This study represents the first study demonstrating 100% protection against vaginal SHIV challenges with an antiretroviral intravaginal ring. Additional pharmacokinetic data were presented in the poster session (Smith, abstract 986).
CROI: A Tenofovir Disoproxil Fumarate Intravaginal Ring Completely Protects Against Repeated SHIV Vaginal Challenge in Nonhuman Primates - (03/11/13)
CROI: Tenofovir in Intravaginal Ring Protects 6 of 6 Macaques From SHIV Challenge - written by Mark Mascolini - (03/08/13)
Themed discussion. Additional discussion of new long-acting antiretrovirals occurred in a packed-room themed discussion Monday afternoon on new long-acting agents (http://webcasts.retroconference.org/console/player/19414?mediaType=podiumVideo), including an overview by Kimberly Struble from the US FDA. Long-acting antiretrovirals have great potential for improving adherence and health benefits both treatment and prevention, and the energy devoted recently to developing delivery technologies (nanosuspensions for injection, slow-release rings, etc.) is encouraging.
Prevention of hepatitis B virus (HBV) and herpes simplex virus type 2 (HSV-2) infections. In a session dedicated to advances in hepatitis therapy, a surprising prevention oral abstract was presented (Brinkman, abstract 33). In a retrospective cohort study among 2942 HIV-infected persons attending an HIV care clinic in the Netherlands, the effect of hepatitis B-active antiretroviral therapy on prevention of primary hepatitis B infection was explored. Data were available on HBV vaccination and infection status and HIV treatment history (particularly HBV-active medications, specifically TDF, 3TC, and FTC). At baseline 30% (n=871) were HBV susceptible, of whom 35 (4%) became infected with HBV. The timing of HBV infection was not known with high precision in this clinical cohort, but the authors were able to test archived samples to better define timing of infection for a number of cases. HBV-active HIV treatment was associated with significantly decreased risk of HBV infection, compared with no HIV treatment or treatment with ART not active against HBV, and TDF-containing regimens were associated with greater reduced risk compared with non-TDF-containing regimens that were active against HBV (i.e., FTC or 3TC alone). Most cases of HBV infection were asymptomatic, and when the 35 cases of HBV infection were explored further, it appeared that no cases of HBV occurred while on TDF (although most TDF exposure included either 3TC or FTC as well, suggesting most TDF exposure was dual-active HBV therapy). Thus, these results suggest that HBV-active ART is protective against HBV acquisition - essentially acting as HBV PrEP - with TDF-containing therapy potentially more effective for HBV prevention than regimens containing only 3TC or FTC.
In a poster (Celum, abstract 999), data from the Partners PrEP Study were explored to understand whether TDF and FTC/TDF PrEP migh protect against HSV-2 infection. In CAPRISA 004, in addition to the prevention effects of tenofovir vaginal gel against HIV, vaginal PrEP, with tenofovir, also reduced the risk of HSV-2 acquisition (by 51%), a surprising finding. Tenofovir has activity against HSV-2 in vitro but the EC90 is high, higher than achieved by oral TDF dosing but not by vaginal gel delivery of tenofovir, potentially explaining the CAPRISA 004 results. In iPrEx, in which FTC/TDF adherence was moderate, no protection against HSV-2 was found (reported at CROI 2012). In the Partners PrEP Study, 1522 participants (36% of the entire population) were HSV-2 seronegative at study entry - 132 seroconverted to HSV-2 during the study: 43 assigned TDF, 37 assigned FTC/TDF, and 52 assigned placebo, translating to 21% (p=0.3) efficacy for TDF, 35% (p=0.05) efficacy for FTC/TDF, and 28% (p=0.06) efficacy when the TDF and FTC/TDF arms were combined. Additional serologic testing is ongoing in this study to better assess potential HSV-2 PrEP protection by TDF and FTC/TDF.
Clearly, additional studies of HBV and HSV-2 PrEP effects, and these results do not obviate the need for HBV vaccination and HSV-2 behavioral risk-reduction in at-risk persons, but they provide great encouragement that effective PrEP therapies might have multiple benefits.
HIV testing and linkages to care
One key area for implementation of combination HIV prevention is achieving high coverage of HIV testing, which is the "gateway" to all the subsequent steps in the HIV treatment and prevention cascades, particularly initiation of ART for those found to be HIV-infected. Two abstracts related to HIV testing were presented as part of the Monday oral session.
Project Accept / HPTN 043 (Coates, abstract 30). Project Accept (HPTN 043) was a 48 community cluster randomized trial of community-mobilization and community-based voluntary counseling and testing (VCT) versus standard VCT. The goal of the trial was to test whether community mobilization to increase awareness of HIV testing and the benefits of knowledge of HIV serostatus coupled with increased access to HIV testing and post-test services would result in reduced risk behaviors and HIV incidence. The study was a tremendous endeavor: pilots began in 2001, the trial was formally initiated in 2004, and the final evaluation - a community random sample with extensive behavioral questions and blood samples later tested as in an innovative laboratory algorithm to evaluate HIV incidence - was done in 2011. The primary outcome of the study was HIV incidence, evaluated at the community level (i.e., regardless of whether someone had actively participated in the HIV testing program), measured in a probability sample of 54,326 residents aged 18-32 years (89% response rate). Project Accept achieved high HIV testing achieved in intervention communities: >15,000 mobilization events, >71,000 HIV tests, >51,000 post-test support services, and community VCT resulted in a 25% overall increase in testing in intervention communities (p=0.003), which was greatest in men (45% increase over control communities). Overall, HIV incidence was reduced by 14% in intervention communities compared to control communities (95% CI -2-27%, p=0.08), and subgroup analyses suggested perhaps greater HIV effects in those 25-32 years of age, particularly women. Reductions in sexual risk were seen and were greatest in HIV-infected men. Importantly, there was no increase in violence related to increased HIV testing. In summary, Project Accept demonstrates the ability to do large complex population-based studies and to do them well. The HIV incidence reduction from mobilization around HIV testing alone was modest, and lower than hoped for in the design of Project Accept. Nevertheless, the results of this important trial give hope that increased HIV testing, coupled with additional behavioral and biomedical interventions (male circumcision, initiation of antiretroviral therapy [ART], PrEP), could have greatest impact.
Approaches to HIV testing (McNaughten, abstract 31). A complimentary approach to achieving high coverage in HIV testing is to find the most effective strategies to provider-initiated HIV testing. McNaugten et al. presented results from a cluster randomized trial of outpatient models for HIV testing in Africa, called Project Status, to evaluate different strategies for provider-initiated HIV testing. 36 outpatient departments in South Africa, Tanzania and Uganda were randomized from October 2011 to June 2012 and persons ages 18-49 and not previously tested were evaluted. The clinics were randomized to three models of HIV testing: on-site referral to HIV VCT (Model A), healthcare provider offer and provision of VCT (Model B), and lay counselor VCT (Model C). Of the 35,843, age-eligible adults attending the study clinics, 45% were tested for HIV. All three models achieved high rates of HIV testing (44% Model A, 37% Model B, 58% Model C). The highest proportion tested for HIV was in the model in which testing was provided at the clinic by dedicated lay VCT staff. Thus, the model of having clinical areas for testing with a health talk and HIV information in the waiting room, with designated staff for HIV testing was the most effective, efficient and convenient. Linkage to HIV care among HIV-infected persons was high: ~94% of the HIV-infected persons linked to care, with an HIV prevalence of ~10% among those tested.
HIV self-testing in Malawi (MacPherson, abstract 95LB, in session http://webcasts.retroconference.org/console/player/19426?mediaType=podiumVideo). An extremely innovative cluster randomized trial including HIV self-testing and home initiation of ART was reported. In 14 urban neighborhoods in Blantyre (total population 16,660, prevalence 18%), HIV self-testing kits were made available through community counselors; 7 neighborhoods were randomized to facility-based HIV care alone (control arm) and 7 were randomized to facility-based HIV care plus the option for home-based initial HIV assessment and ART initiation (intervention arm). The trial included a 6 month intervention period and a 6 month period of follow-up. Overall uptake of self-testing was 58% of adults (65% in intervention, 53% in control). ART initiation was substantially higher in the intervention arm: 2.2% of adults vs. 0.7%, RR 2.94, 95% CI 2.10-4.12). The entire difference in ART initiation was as a result of the optional home initiation of ART. HIV disclosure was also higher in the intervention arm compared to the control: 6.0% vs. 3.3% (RR 1.86, 95% CI 1.16-2.97). This study demonstrates high demand for HIV self-testing and that home assessment by a nurse for ART initiation (as an example of proactive engagement in care soon after learning of a positive HIV result) is a viable and successful strategy.
Finally, a symposium discussion reviewed new opportunities and challenges with HIV self-testing and also provided in-depth information about innovative methods for cross-sectional assessment of HIV incidence, such as that used in Project Accept/HPTN 043 (http://webcasts.retroconference.org/console/player/19452?mediaType=slideVideo).
Men who have sex with men
A Tuesday plenary by Chris Beyrer on the global HIV epidemic among men who have sex with men provided an excellent overview of a too-long neglected topic (http://webcasts.retroconference.org/console/player/19422?mediaType=podiumVideo).
In the Tuesday oral abstract session, HIV prevalence and knowledge of serostatus in an ongoing venue-based sampling study from 20 US cities was reported (Wejnert, abstract 90). For all ages and races/ethnicities, there was a significant increase in knowledge of self-reported HIV serostatus between 2008 and 2011. Black men who have sex with men were 40% less likely to be aware of HIV infection than white men who have sex with men, and HIV prevalence was highest among black men. Thus, encouragingly, there appears to have been a substantial generalized change in HIV awareness across all men who have sex with men, reflecting likely a combination of knowing one's HIV status and willingness to disclose to interviewers. Black men who have sex with men are a priority population for prevention efforts in the US.
A themed discussion on men who have sex with men
(http://webcasts.retroconference.org/console/player/19430?mediaType=podiumVideo) included additional abstracts of interest.
US epidemiology
A symposium on preventing HIV in the US (http://webcasts.retroconference.org/console/player/19419?mediaType=podiumVideo) brought together four excellent presentations about whether we can do better. Ada Adimora discussed cultural, social and structural issues which are factors in the geographic and other disparities in HIV prevalence and risk, including 'ecosocial' networks. Steven Goodreau discussed epidemiologic analyses and mathematical modeling to estimate the proportion of infections arising from acute vs. chronic infection. Math modeling indicates that acute infection accounts for a higher proportion of infections in the context of multiple, short relationships (which has been reported in some populations of men who have sex with men) or multiple concurrent partnerships (which has been described in some African heterosexual populations). His stochastic, dynamic modeling indicates that a significant minority (36%) of transmission in MSM in the Americas occur in main partnerships. Carlos del Rio assessed the goals of the US National AIDS strategy, including to reduce new infections by 25%, decrease the prevalence of HIV, scale up knowledge of HIV serostatus. He described the importance of multi-component HIV prevention strategies: 1) testing for those unaware of status; 2) linkage to care and retention in care; and 3) counseling about risk reduction in order to reduce risk behaviors and increase the proportion with viral suppression. He highlighted the importance of targeted prevention interventions among populations with greatest number of new infections (e.g., men who have sex with men); focused prevention efforts in high prevalence cities (e.g., through ECHPP, Enhanced Comprehensive HIV Prevention planning project) in 12 U.S. MSAs/cities with the highest HIV prevalence; and high coverage of evidence-based interventions (e.g, treatment as prevention). He prioritized the importance of overcoming barriers to routine testing, given data that indicate that 18% with undiagnosed HIV are associated with 50% of sexual transmission. He also encouraged implementation of partner services of HIV-infected persons and couples testing, and to find effective strategies for clinical settings to modify behavior and increase retention in care and ART adherence. Regarding PrEP, he recommended striving for lower costs and higher efficacy. Regarding ART impact, he recommended increased use of generic antiretrovirals and improved linkage through surveillance systems and electronic medical records. Alan Greenberg addressed the ART cascade, and reported that higher proportions have viral suppression in the Veterans Administration (50%) and health maintenance organizations with 61% viral suppression in Kaiser patients. He reminded the audience that similar populations are at risk of not linking and not being retained in HIV care, once linked. He supported the standard cascade-related process measures as recently recommended by IOM. He described innovative efforts to achieve higher linkage and retention, such as in Washington DC, in which a 'red carpet program' has been implemented for DC residents who have been newly diagnosed with HIV or returning to HIV care; no insurance is required, a clinic appointment is scheduled within 1-2 days; and a patient-centered medical 'home' is developed to coordinate care and provide results to patients, which is consistent with the Affordable Care Act objectives.
Prevention of mother-to-child transmission of HIV
Prevention of mother-to-child transmission (PMTCT) of HIV is a tremendous public health achievement. The CROI 2013 N'Galy-Mann lecture was given by Lynne Mofenson, from the Eunice Kennedy Shriver National Institute of Child Health and Development, who has been a global leader in HIV prevention in women and children. The talk is a tour de force, covering mother-to-child prevention from the beginning of the epidemic and thinking forward to the possibilities and challenges of eradication of pediatric HIV:
http://webcasts.retroconference.org/console/player/19405?mediaType=podiumVideo
A Tuesday oral abstract session was dedicated to progress in PMTCT of HIV infection ( http://webcasts.retroconference.org/console/player/19425?mediaType=podiumVideo).
In Malawi, Option B+ (i.e., initiation of ART in pregnant women and continuing for life, regardless of initial CD4 count) was implemented starting in July 2011. Since that time, the number of women receiving ART during pregnancy has increased, with 87% of known HIV-infected pregnancy women receiving ART in the third quarter of 2012 (Barr, abstract 82). ART retention was 83% at 6 months. These results indicate early support and enthusiasm for this aggressive program.
For countries without aggressive maternal ART programs, infant prophylaxis (analogous to PrEP) during breastfeeding has been shown to decrease HIV risk. At CROI 2013, the results of HPTN 046, a randomized trial of 6 weeks versus 6 months of daily infant nevirpine prophylaxis in HIV-exposed and breastfed infants were reported (Fowler, abstract 84LB). Perhaps not surprisingly, HIV infections after 6 weeks were reduced in those infants who had prophylaxis extended to 6 months compared to those who stopped at 6 weeks (1.1% vs. 2.4%, p=0.05). However, this difference was not sustained after prophylaxis was stopped and there were no significant differences in HIV at 9, 12, or 18 months. Like for PrEP in adults, these findings emphasize that prophylaxis works but only when used when exposure is ongoing.
Finally, of course, the widely-publicized case of a single infant from the US apparently cured of HIV infection after receiving combination ART early in life received considerable attention. The abstract (Persaud, abstract 48LB) was presented as part of a session on HIV eradication (http://webcasts.retroconference.org/console/player/19411?mediaType=podiumVideo).
Male circumcision
A themed discussion on Monday highlighted the implementation and cost-effectiveness of voluntary medical male circumcision for HIV prevention, featuring 5 posters (abstracts 1007-11)
(http://webcasts.retroconference.org/console/player/19415?mediaType=podiumVideo). These posters focused on key aspects of male circumcision implementation, which has proven to be challenging in many parts of sub-Saharan Africa.
Critical aspects to increasing male circumcision uptake include demand stimulation for different ages and populations; the role, safety and acceptability of devices; models of delivery of male circumcision (urban vs. rural, mobile camps vs. facility based, and doctor vs. nurse provision); strategies to incorporate male circumcision into traditional male circumcision and rite of passage strategies; and feasibility of infant male circumcision.
In poster 1007, Kanyago and colleagues reported a comparison of the Shang ring to standard forceps-guided adult male circumcision in a randomized trial in western Uganda of 138 men. They reported a shorter procedure time, a higher rate of adverse events (e.g., slippage of the ring, infection and any complication by day 14), higher proportion that returned to activity by day 1, and higher patient satisfaction with the Shang ring.
In an analysis of the large-scale implementation of medical male circumcision in Nyanza province from 2008-2011 (poster 1008), Chesang reported the incidence of intra-operative and post-operative adverse events reduced by 20-fold and 4-fold, respectively; nurses had fewer adverse events than other cadres of health workers. In multivariate analysis, fewer adverse events were associated with the number of male circumcisions performed, program maturity > 2 years, targeting 15-19 year-old men, using dedicated staff and task shifting to nurses.
In poster 1009, Kong and colleagues from the Rakai program, reported on coverage since 2007 after the clinical trial was completed. Free male circumcision was offered to men ≥ 13 years of age in one central facility and four satellite rural clinics; since 2011 mobile camps were conducted with outreach programs. The prevalence of male circumcision among non-Muslim men increased from 12% in 2006 to 28% with 4% annual increase in the context of 97% of uncircumcised men reporting that MC confers benefits and knowing where to get circumcised. Thus, demand generation will be needed, particularly among groups most at risk, in order to reach the WHO/UNAIDS goal of 80% male circumcision by 2016.
In poster 1010, Kacker presented on health economic implications of male circumcision scale up for the prevention of HIV and other STIs, using Rakai cohort data and using microsimulation modeling of four alternative scenarios of scale-up: gradual or rapid with adult/adolescent only or infant male circumcision as well as adult/adolescent male circumcision. The investigators considered costs, AEs, and reduction in HIV and STIs. Rapid male circumcision scale-up, including infant male circumcision, was found to be cost saving within 10-15 years. Prior analyses may have significantly underestimated cost savings; non HIV STIs contributed to the benefits and accounted for 50% of cost savings within 10 years.
In poster 1011, Plank and colleagues reported on maternal motivators and barriers to the uptake of neonatal male circumcision as part of HIV prevention efforts in Botswana. Mothers of newborn boys in three Botswana maternity wards were invited to answer questionnaires on knowledge, attitudes and acceptability of neonatal male circumcision in 2009-10. Of the 547 women who completed the questionnaire, 35% reported they were HIV-infected and 93% said they would want neonatal male circumcision and 55% brought their infant for male circumcision. The predictors of uptake of neonatal male circumcision were knowledge that male circumcision protects against future HIV acquisition; hygienic, cultural or religious reasons to circumcise; and father's participation in the decision about male circumcision for the infant.
Hormonal contraception and HIV risk
Hormonal contraception is used widely and plays an important role in preventing unintended pregnancies and reducing maternal morbidity and mortality. However, some, but not all, prospective observational studies have found an increased risk for women to acquire HIV infection when they are using hormonal contraception, especially injectable depot medroxy-progesterone acetate (DMPA, otherwise known as branded Depo-Provera). This injectable contraceptive is currently the most popular contraceptive method used in southern and East Africa as it is easy to use, fast to administer, and can be used discretely. For women in regions where HIV rates are high and injectable contraceptive use is common, such as southern and East Africa, understanding the possible link between injectable contraceptive use and HIV infection is a public health priority. The challenge with studies on this issue is that observational data are potentially open to bias, and hormonal contraceptive users could be less likely to use condoms (and, even more importantly, less likely to use condoms but more likely to falsely report using them), which could result in confounding in the analysis.
At CROI 2013, data from another large prospective observational study were reported (Crook, abstract 28, during the Monday oral session). Data were a secondary analysis of the MDP 301 trial, a phase III multisite trial of the microbicide gel PRO2000 conducted between 2005 and 2008. A total of 9385 women were enrolled in South Africa, Uganda, Tanzania, and Zambia. Follow-up was 52 weeks. PRO2000 gel was found to be ineffective for HIV prevention in the study population. The secondary contraception and HIV risk analysis included 8663 women who had follow-up, were <50 years of age, and were not using contraceptive implants (due to small numbers). Contraception was recorded every 4 weeks: the comparison groups were DMPA (29% of women at baseline), NET-EN (another progestin injectable methods, 14% of women at baseline), oral contraceptive pills (16% of women at baseline), and women using no hormonal contraception. HIV testing was at weeks 12, 24, 40, 52 and there were 382 HIV seroconversions. HIV incidence was 5.9, 6.0, 3.5, and 3.5 for DMPA, NET-EN, oral pill, and non-contracepting women. After adjustment for potential confounding factors, DMPA was associated with a modest but statistically significant increased risk of HIV acquisition (adjusted HR 1.36, 95% CI 1.06-1.77), with no statistically significant increased risk for NET-EN or oral contraceptive pill users. In sum, there was a small increased risk associated with DMPA. This analysis represents the largest single study to date of DMPA and HIV risk, although these results are still potentially susceptible to confounding. Data for NET-EN are more limited, although results from this analysis are encouraging that NET-EN's HIV risk may be different than that suggested for DMPA. Clearly, more data and interpretation are needed to definitively answer this important public health question.
In a second contraception-HIV abstract in this session, the relationship between DMPA use and genital HIV shedding in HIV-infected women from Kenya who were taking ART was evaluated (Day, abstract 29). Limited data have suggested that, in addition to potentially increasing HIV susceptibility of uninfected women, DMPA might increase HIV transmission risk among HIV infected women. The results reported at CROI 2013 suggest that successful combination ART overcome any DMPA effects on genital HIV. The study presented evaluated 102 Kenyan HIV-infected women who were followed prospectively every 3 months. Median CD4 count at ART initiation was 122 (IQR 78-164). Women provided a median of 12 follow-up visits, over median 34 months. Plasma HIV was <400 c/mL at 85% visits, and cervical HIV was undetectable at 94% of visits. DMPA not associated with detection of HIV in plasma / endocervical secretions in these women who appeared to be highly adherent to ART. These data are reassuring that for women on ART, with high adherence, DMPA may not increase genital HIV shedding.
A valuable themed discussion on fertility for HIV-infected and at-risk women discussed several important abstracts (http://webcasts.retroconference.org/console/player/19431?mediaType=podiumVideo).
Finally, an excellent symposium on hormonal contraception and HIV risk, from biologic studies to review of the epidemiologic literature to mathematical modeling of potential public health impact, occupied late Tuesday afternoon and is worth watching in full (http://webcasts.retroconference.org/console/player/19435?mediaType=podiumVideo).
|
| |
|
 |
 |
|
|