 |
 |
 |
| |
Hepatitis C Report from AASLD: HIV/coinfection; new HCV drugs
Written for NATAP by Andrew Talal, MD, Cornell-NY Hospital, GI and Hepatology Clinic
|
| |
| |
Topics:
--Treatment in HCV/HIV coinfected patients
--why coinfected patients may have impaired response to HCV
--new drugs in development for HCV: histamine, ISIS 14803 (antisense inhibitor), IL-12, RBV substitutes (levovirin, viramidine heptazyme VX-497)
--effect of interferon therapy on fibrosis progression and development of hepatocellular carcinoma
--hit hard & hit early with HCV therapy??
A. Treatment of Coinfected individuals
Interferon A-2B/Ribavirin combination therapy in HIV/HCV coinfected persons:
Results of a Multicenter Randomized, Double-Blind, Controlled Trial (AmFAR Study). Abstract 634
BKG/objectives: There have been a few published studies using combination IFN/RBV in coinfected patients. The objective of this double-blind study was to compare IFN/RBV combination to IFN monotherapy in coinfected individuals.
Methods: 110 coinfected individuals were randomized to receive IFN-a-2b (3 MU TIW) and RBV (400 mg BID) or IFN-a-2b (3 MU TIW) for 48 weeks. Patients with HCV RNA detectable at week 12 were given RBV at week 16. Baseline characteristics were balanced between the two groups. Genotype 1 was present in 73% of the patients, 84% had fibrosis, all had inflammation, 15% had cirrhosis, 59% had necrosis, and median peripheral CD4 count was 504 cells/mm3 (12-1195).
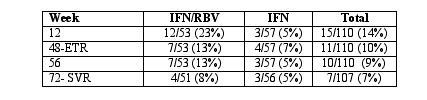
Results: 12/53 (23%) of the patients who received IFN/RBV and 3/57 (3%) of the IFN monotherapy group achieved HCV RNA below assay detection at week 12 (p = 0.016). The efficacy data are presented in the Table below:
|
|
| |
 |
|
| |
Fifty-four percent of the individuals discontinued study medications before week 48. Twenty percent reported that the discontinuation was secondary to adverse events. Frequent reasons for discontinuation included elevated LFTs, hypertriglyceridemia, leukopenia, anemia, and anesthenia.
Conclusions/Commentary: RBV/IFN combination is more effective than IFN monotherapy at decreasing HCV RNA below assay detection by week 12 after therapy initiation. The low efficacy rate of these medications in this population, largely impacted upon by the large number of patients who discontinued prematurely, may be more reflective of this particular study. We will have to await data from additional studies to determine if HCV treatment efficacy is substantially reduced in coinfected individuals.
Safety and efficacy of 40Kda Peginterferon Alfa-2A (Pegasys) in the treatment for patients coinfected with HIV and HCV: Preliminary results from a randomized, multicenter trial. Abstract # 623
BKG/objectives: The aim of this study is to investigate the safety and efficacy of 48 weeks of pegylated interferon-a-2a plus 34 weeks of ribavirin or ribavirin placebo in HIV/HCV coinfected patients in a controlled and partially blinded randomized study.
Methods: 150 HIV/HCV coinfected individuals were treated with 180 mcg SC qwk (once weekly) x 48 wks. Subjects who did not achieve HCV RNA below assay detection or > 2 log decrease in HCV RNA at week 12 were randomized to receive RBV 800 mg/d or RBV placebo. After week 24, all patients with detectable HCV RNA discontinue therapy. For analysis purposes, there were three arms to the study:
1) Arm A: 12-week responders who continue treatment with peg-IFN-a-2a
2) Arm B: 12-week nonresponders who continue treatment with peg-IFN-a-2a plus RBV
3) Arm C: 12 week nonresponders who continue treatment with peg-IFN-a-2a plus RBV placebo.
Editor's notes: Patients could have CD4s as low as 100 for this study and detectable HIV-RNA. 106 patients enrolled; age 44; 82% male (women respond better); ALT 101; 59% had high viral load (6 million); average HCV-RNA 6.3 log IU/mL; 80% genotype 1 (this is a high percentage of genotype 1); average HIV-RNA 2.3 log; 11% cirrhotic. Cd4 at baseline was 513 and 436 at week 12. 63% had <50 copies/ml HIV-RNA at baseline and 72% at week 12.
Results: 33.7% (30/89) of patients achieved a > 2 log10 decline in HCV RNA from baseline to Week 12. 19.1% (17/89) patients were HCV RNA negative at week 12. Nine (8.5%) patients withdrew from the study, 5 (4.7%) as a consequence of expected adverse events, 4 (3.8%) as a result of failure to keep appointments or refusal to comply with therapy. Out of a total of 468 adverse events that were reported, only one (bacterial pneumonia) was serious.
Conclusions/Commentary: These are preliminary results that demonstrate that peg-IFN-a-2a is efficacious in coinfected patients with a side effect profile that is similar to that reported for studies in HCV monoinfected individuals. We anxiously await data on the SVR that is seen in coinfected patients who are treated with peg-IFN-a-2a and RBV.
Editors Comments: In previously reported Pegasys monotherapy studies, the range of SVRs in these studies were 28% to 38%. Perhaps the response in this study will improve by week 24 since 33% had >2 log reduction. We anxiously await these forthcoming results.
Safety and efficacy of interferon alfa-2B and ribavirin combination therapy for the treatment of hepatitis C in patients coinfected with HIV. Abstract #653
BKG/objectives: To determine the safety and efficacy of IFN (3 MU TiW) and RBV (800-1200 mg qd) in coinfected individuals.
Methods: HIV/HCV coinfected and HCV monoinfected patients were matched 2:1 on genotype, HCV RNA and presence of cirrhosis. Treatment duration 24 weeks in patients with genotype 2 or 3 and 48 weeks in patients with genotype 1. (Genotype 1 patients with detectable HCV RNA at Week 24 were withdrawn).
Results: There were no significant differences between the two patient groups with regard to age, race, gender, risk factors for HCV, body weight, ALT, WBC count, hemoglobin concentration, or platelet count. There were no significant adverse events or deaths in either group.
|
|
| |
 |
|
| |
Conclusions/Commentary: This study, although it includes small numbers of individuals, demonstrates that the SVR in coinfected individuals is not significantly different from that in HCV monoinfected individuals.
B. Novel agents for HCV treatment
A four week trial of VX497 (an IMPDH inhibitor) combined with interferon in previously untreated patients with chronic hepatitis C. Abstract #628
BKG/objectives: IMPDH inhibitors (which is the same class of drugs to which RBV belongs) act by inhibiting an essential enzyme in the de novo purine biosynthesis pathway (a pathway that is responsible for making substances that are important for the proper functioning of many body functions). These agents have in vitro activity against +-stranded RNA viruses, such as hepatitis C virus. The aim of this study was to assess the four-week safety and tolerability of the combination of VX497 (a new IMPDH inhibitor) and IFN-a in treatment naive HCV infected individuals.
Methods: This study was randomized, double-blinded, placebo controlled in which participants received one of three doses of medication;
1) IFN-a 3MU SC TIW + VX497 (100 mg PO TID), n = 17,
2) IFN-a 3MU SC TIW + VX497 (300 mg PO TID), n = 18,
3) IFN-a + placebo, n = 18.
Patients were treated for 28 days and then Rebetron was added to the regimen to complete 12 months of therapy.
|
|
| |
 |
|
| |
There was a significant difference in HCV RNA levels at baseline between the three groups. However, when adjusted for differences in baseline HCV RNA, the antiviral effect of group 1 vs. group 3 indicated a statistical trend in favor of group 1 (p = 0.1)
The conclusions that can be drawn from this study are that there was no increase in toxicity using the combination of VX497 and IFN when compared to the group that received only IFN. The study demonstrated a trend toward enhanced antiviral effect at 4 weeks with VX497 when compared to those who received IFN and placebo. Based on these results, further studies are planned. However, it is interesting to note that the 100 mg dose showed more of an effect than the 300 mg dose. Whether this is simply due to insufficient numbers of subjects to demonstrated a statistical difference or whether this is due to a lack of an effect of the medication will need further investigation.
Phase I clinical studies of levovirin- a second generation ribavirin candidate. Abstract #620
Levovirin is an isomer of ribavirin with similar immunomodulatory properties (ability to modulate the immune system). In animal studies, the medication has decreased toxicity in comparison with that observed with RBV. The aim of this study was to characterize the safety and PK profiles of levovirin in humans.
In this study, oral doses of 200, 400, 600, and 1200 mgs were administered to healthy volunteers (n = 6 at each dose and 2 on placebo per dose group). Subjects who received 200 mg subsequently received the 600 mg dose and those in the 400 mg group subsequently received 1200 mg.
A total of 16 patients participated in the trial. Levovirin was orally absorbed, peaked at a consistent Cmax (a measuse of how soon after the drug is given one sees the maximum concentration in the body) of 4 hours, and was excreted unchanged in the urine. There were very strong correlations between the maximum concentration and levovirin dose (R2 = 0.97) and between AUC (another measure of how long the drug persists in the body) and levovirin dose (R2= 0.98).
This study shows that a levovirin dose up to 1200 mg is well tolerated in humans, and that there was strong linearity between Cmax and AUC with levovirin dose. This product, like several of the other products that I have commented on, may be useful in the treatment of chronic HCV. Further studies must be completed until we will know about the efficacy of this therapy in humans.
Clinical pharmacokinetics of ISIS 14803, an antisense inhibitor of HCV, in patients with chronic HCV and comparison to non-clinical pharmacokinetics/toxicology in monkeys. Abstract #636
This compound (a 20-base, phosphorothioate oligodeoxynucleotide) inhibits HCV replication and protein expression in cell culture and mouse models (through an antisense mechanism). The compound, which has a high concentration in the liver, functions by the disruption of viral RNA (by RNase H). The goal of this trial was to assess the pharmacokinetics of the compound administered by intravenous infusion and subcutaneous injection in patients with chronic hepatitis C.
In this 32-day trial, the compound is administered TIW. ISIS 14803 was measured in serum samples obtained prior to the first dose and after the last dose (d 32).
In this trial, three different doses were tested. The results demonstrated that the compound does not accumulate in plasma with repeated dosing and that this compound behaves in a similar fashion as other members of the same class of drugs (phosphorothioate oligodeoxynucleotides) that have been tested in humans. The drug has a high concentration in the liver with a half-life of four days, which means that the drug can be given once every other day.
This study demonstrates that this compound may be effective in the treatment of HCV and is going to be tested in larger trials involving humans. This study was a non-clinical pharmacokinetics/toxicology study in monkeys to establish the kinetics of the drug. Further data should be forthcoming shortly. Although a few patients who were treated with this compound had transient increases in ALT (as presented in abstract 712), clinical trials using this medication are proceeding.
See small preliminary study below reporting on antiviral activity and safety in humans.
Safety analysis of a phase I study of heptazyme, a nuclease resistant ribozyme targeting hepatitis C (HCV) RNA. Abstract #646
BKG/objectives: Ribozymes are catalytic RNA molecules designed to cleave specific RNA sequences. Ribozymes have been stabilized to resist enzymatic and chemical degradation. Ribozyme cleavage of RNA in cells results in a reduction of the RNA available for translation, a reduction in viral protein synthesis, a reduction in minus-strand RNA intermediate, and a reduction in nascent plus strand RNA. The ultimate result would be a decrease in the number of virus particles that are detectable in the peripheral blood. Heptazyme, a particular form of ribozyme, has been designed to cleave the HCV 5'-UTR and can inhibit virus replication in vitro in an HCV-polio virus chimera. The goal of the current trial was to evaluate the safety of Heptazyme in individuals with chronic HCV infection.
Methods: Prospective, 28-day trial in patients proscribed one of four doses of Heptazyme:
A- 3 mg
B- 10 mg
C- 30 mg
D- 90 mg
Results: 19/24 subjects reported a total of 39 adverse events that were rated as possibly or probably related to Heptazyme. Gastrointestinal side effects and changes in emotional state were particularly common.
Conclusions: Heptazyme was safe and well tolerated and a phase II study using the medication in patients with chronic hepatitis C has been arranged in combination with interferon.
Effect of interferon and ribavirin on progression of liver fibrosis in patients with severe chronic hepatitis C. Abstract #625
The goal of this study was to assess the impact of treatment with IFN/RBV combination therapy on progression of hepatic fibrosis in patients with chronic hepatitis C.
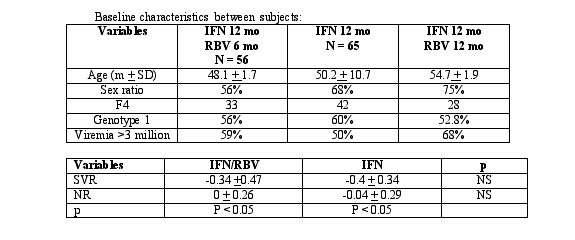
In this study, 200 chronically HCV-infected individuals (all with Knodell fibrosis score 3-4) were enrolled in two clinical trials between 1996-1998. Group A-75 individuals received 12 months of interferon monotherapy and ribavirin for six months. Group B- 75 individuals received 12 months of interferon monotherapy and Group C- 50 individuals received combination therapy of interferon and ribavirin. Groups A & C were comparable in terms of age, ALT, HCV RNA, genotypes, and liver histology according to Knodell. There were more males in group C (75%) compared to group A (56%) (p = 0.053). The fibrosis progression rates were calculated (fibrosis score/number of years with HCV infection or fibrosis score/number of years between biopsies).
|
|
| |
 |
|
| |
Rates of fibrosis progression were compared in seventy-six patients with liver biopsies before and after treatment. The mean fibrosis progression rates prior to treatment were 0.26 in the group that received interferon and ribavirin and 0.212 in the group that received interferon monotherapy. After treatment, the mean fibrosis scores were -0.141 in the group that received combination therapy and -0.127 in the group that received interferon monotherapy. There was no significant difference in the fibrosis rate in those treated with combination therapy compared to those treated with interferon monotherapy, while there was a difference in those who achieved a virologic response compared to those who did not (p < 0.002). This study demonstrates that a virologic response is the best predictor of the inhibition of fibrosis progression and that both interferon and ribavirin as well as interferon monotherapy are both able to decrease fibrosis progression in individuals with chronic HCV infection.
Editorial Comment: this is one of several studies reported on at AASLD showing improved fibrosis following treatment.
More details on this study are available; it appears nonresponders also slowed progression; the authors suggested that in nonresponders it is nor necessary to continue RBV:
www.natap.org/2001/aasld2/day25.htm
Thierry Poynard and a French research group reported similar findings at AASLS: nonresponders could stop progression; that cirrhosis was reversed to some degree in about 50% of patients in the follow-up so far; achieving a sustained response is a significant factor-
www.natap.org/2001/aasld2/day21.htm
Interferon retreatment reduces or delays the incidence of hepatocellular carcinoma in patients with chronic hepatitis C. Abstract #630
The goal of this study was to assess whether the incidence of hepatocellular carcinoma is decreased in virologic nonresponders who are treated with an additional course of interferon (after an initial course of interferon).
To be included in this study, patients had to receive a total dose of at least 250 million units of interferon, to not have achieved a sustained virologic response to interferon, and to have at least two years of follow-up after interferon discontinuation. Radiographic imaging (ultrasound or computed tomography) of the liver was performed every 3-6 months after completion of therapy to detect hepatocellular carcinoma.
Results: Hepatocellular carcinoma developed in 34/210 (16.2%) of patients who did not receive retreatment while it developed in 5/99 (5.1%) of retreated patients (p = 0.01).
Results of Cox proportional hazards model are demonstrated in the Table below:
|
|
| |
 |
|
| |
The multiple regression modeling shows that age, the # of IFN treatments, and response to initial treatment were independently associated with the incidence of HCC in HCV infected individuals who did not achieve an SVR to an initial course of interferon therapy.
Conclusions: This study indicates that retreatment with IFN in HCV-infected individuals who fail to achieve an SVR to an initial course of IFN delays or prevents the development of HCC. Therefore a second course of therapy is warranted in virologic nonresponders.
The hit hard and hit early strategy to treat chronic hepatitis C. Abstract #662
BKG/objectives: The goal of this study was to evaluate whether daily dose interferon would prevent the development of mutations in the hepatitis C virus that may affect its responsiveness to treatment. In this study, daily interferon administration was compared to three times per week.
Methods: 70 therapy-naive chronically HCV infected individuals were treated with (Group A) induction therapy with IFN-a-2a 6 MU daily x 12 wks and (Group B) IFN-a-2a 6 MU TIW x 12 wks. Those individuals who had HCV RNA below assay detection, IFN was tapered to 4.5 MU x 4 wks followed by 3.0 MU x 4 wks. Patients were then treated with IFN 3 MU TIW and RBV 800 mg qd x 6 months.
Results: Treatment efficacy in the two groups is shown in the Table below:
|
|
| |
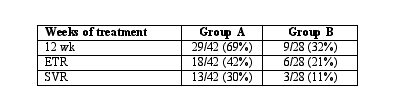 |
|
| |
Conclusions: The higher response rates in the patients who received daily interferon indicates that the hit hard and hit early treatment strategy may be more effective in patients with chronic hepatitis C.
Editorial Comment: this suggests to me that an induction regimen used up front in combination with pegylated interferoon may improve response, but studies will have to be conducted to configure dosing._
A dose-escalation study of ISIS 14803, an antisense inhibitor of HCV, in chronic hepatitis C patients. Abstract 712
Introduction: 20-2' deoxynucleotide N heterodimer O-O linked. Complementary to IRES/translation initiation.
Methods: This is a dose-escalating study in patients with chronic, compensated hepatitis C plasma HCV RNA greater than 10,000 copies/ml. The drug was administered three times per week for four weeks either by 2-hour intravenous infusion or bolus subcutaneous administration.
Results: Two patients, treated with 2 mg/kg had decreases in plasma HCV RNA of 1.4 and 1.5 log that persisted for 30 days post dose administration. Two additional patients, one who received 2 mg/kg and the other who received 0.5 mg/kg, both had HCV RNA reductions of > 0.5 log.
An ALT flair occurred in two of the four patients associated with at least 1.0 log reduction in HCV RNA and in two additional patients, one with a more most decrease in HCV RNA and one without change in HCV RNA. During the transient ALT flair, there was no increase in total bilirubin, albumen, or PT, and a liver biopsy, performed in two of the patients, did not reveal any evidence of drug toxicity. The Table below summarizes the results of this study.
|
|
| |
 |
|
| |
The implications of this study are that two out of four patients treated with ISIS 14803 2 mg/kg had a > 1 log decrease in HCV RNA. Some of these patients experienced nonspecific increases in ALT, which do not appear to be of important clinical consequences. Although preliminary, ISIS 14803 may be a promising therapeutic addition, and clinical trials of this drug are proceeding.
Efficacy and safety of the combination of histamine dihydrochloride and interferon (IFN) alpha-2B in a phase II trial in naive patients with chronic hepatitis C. abstract 713
BKG/Objectives: HCV infection is associated with an increase in intrahepatic oxidative stress that is mediated by intrahepatic phagocytes. Intrahepatic monocytes/macrophages inhibit the increase in immune function that occurs as a result of treatment with interferon. Histamine binds to H2 receptor and prevents synthesis of NADPH oxidase (a key reaction in the immune function). Therefore, histamine protects cells from inhibition and can restore responsiveness to interferon. The aim of this study was to evaluate the feasibility and safety of four dose regimens of histamine in combination with IFN in chronic HCV infection.
Methods: Four doses of histamine dihydrochloride (3 mg/wk, 5 mg/wk, 6 mg/wk, 10 mg/wk) and interferon were tested (editorial note: histamine is administered by subcutaneous injection)
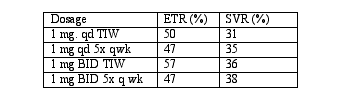
Results: End of treatment responses (ETR) and sustained virologic responses (SVR) (as evaluated by intention to treat analysis) were as follows:
|
|
| |
 |
|
| |
Tolerability: 5% of patients had to discontinue therapy secondary to adverse effects. (editorial note: Injection-related side effects are associated with histamine)
Conclusions: The authors concluded that:
(a) Combination therapy containing histamine and IFN-a-2b appears more effective than IFN-a-2b monotherapy as reported historically.
(b) The data suggest that combination therapy containing histamine have an acceptable safety profile. While these preliminary data appear interesting, the authors have failed to demonstrate a statistically significant improvement with the higher doses and they have not demonstrate that the SVR with histamine is significantly better than with standard therapy alone. Hopefully, if this therapy is studied at all in future trials, histamine will be combined with pegylated interferon and ribavirin to pegylated interferon and ribavirin alone.
Commentary: It appears that the efficacy of IFN combined with histamine is very similar to that of combination therapy with interferon and ribavirin. However, whether the combination of interferon and histamine offers any advantage over combination therapy with interferon and ribavirin remains to be determined through additional clinical trials that are currently planned. The same study was presented at ICAAC abs #H751.
Editorial Comments: the subcutaneous injections and injection-related side effects are limitations of the drug.
A phase II randomized, double-blind, placebo-controlled multicenter study of recombinant human interleukin-12 for the treatment of chronic hepatitis virus infection in patients non-responsive to previous treatment with interferon-alpha with or without ribavirin. Abstract 714
Introduction: Interleukin (IL)-12 is a cytokine produced by mononuclear phagocytes that is a mediator of the innate immune response to intracellular pathogens and is a key inducer of cell-mediated immunity. The cytokine activates natural killer cells, promotes IFN-g production by NK and T cells, enhances the cytolytic activity of NK cells and CTLs, and promotes the development of TH1 cells (all of these are immune effects that are important in the defense against the virus). The aim of this study was evaluate the efficacy and safety of recombinant human IL-12 in the treatment of chronic HCV infection in patients who did not respond to a previous course of treatment with interferon with or without ribavirin.
Methods: Patients were randomized to receive 500 mg/kg rhIL-12 or placebo (blinded) subcutaneously twice weekly for 12 weeks. After the initial 12 weeks, the study was unblinded and patients who were randomized to receive IL-12 received an additional 36 weeks of therapy and those who had received placebo received a total of 48 weeks of IL12. Entry criteria required HCV RNA > 104 c/ml (NGI), elevation of ALT >1.25x ULN, and liver biopsy (within 12 mo) demonstrating chronic hepatitis. Follow-up HCV RNA, ALT and liver biopsy obtained 12 weeks after medication discontinuation.
Results: 289 patients were screened, 225 were enrolled. A total of 160 patients completed at least 8 weeks of rhIL-12. Histologic response: Of 50 of 225 patients with matched liver biopsies, 20/50 (40%) had improved HAI score of greater than 2 with a mean improvement of 4.9 points. The HAI was worse in 20 patients and unchanged in 8 patients. Adverse events: Most patients experienced flu-like symptoms including chills, fever, fatigue, headaches, and arthalgias. Other adverse events include stomatisis (n = 68), peripheral edema (n = 24), hematologic toxicities including profound cytopenia (n = 2), ascites (n = 1), depression requiring hospitalization (n = 1), melena (n =1). (editorial note: Platelets and hemoglobin were reduced)
Conclusions: Because of a lack of efficacy (only 2/160 [1%] achieved SVR) and 3% suffered adverse events thought to be secondary to rhIL-12, the study was terminated early.
Comments: This study joins the list of agents that have been evaluated for the treatment of nonresponders to IFN therapy. It is likely that there will be several more until we are able to achieve another therapeutic breakthrough as the immunological response to HCV infection is likely to be very complicated.
Immunomodulatory activities of viramidine, a liver-targeting ribavirin prodrug, in vitro and in vivo. Abstract 715
BKG/Objectives: A portion of RBVs efficacy in HCV is thought to the drugs' ability to foster a TH1 cytokine response that favors antiviral cellular immunity. The side effect profile of RBV, with a large percentage of individuals who are not able to tolerate the drug secondary to anemia, has provided impetus to search for other formulations with the same efficacy but with an improved side effect profile. The aim of this study was to determine whether viramidine, a liver-targeting produrg analog of RBV, has similar in vivo and in vitro immunomodulatory activity.
Methods: The in vitro effect of viramidine on type 1 cytokine production (IL-2, IFN-g, TNF-a) in activated human T cells was measured by ELISA. In vivo activity was measured in a murine model by type 1 cytokine-mediated contact hypersensitivity (CHS) responses to dinitrofluorobenzene (DNFB).
Results: Effect on Th1 cytokines- Both RBV and viramidine can induce IL-2, TNF-a, and IFN-g. A dose response was demonstrated by CHS response in BALB/C mice. Tissue distribution- Based on 14C labeling, viramidine concentration increased three-fold in liver compared to ribavirin. However, the drugs' first pass effect is increased compared to RBV, which results in similar serum levels. Viramidine levels in RBCs are reduced compared to RBV, suggesting perhaps less side effects related to anemia and fatigue. Toxicity studies- Toxicity studies were performed in monkeys, which demonstrated that for equivalent "area under the curve (AUC)" between RBV and viramidine, one half of the dose of viramidine is required.
Conclusions: Viramidine demonstrated similar immunomodulatory properties to RBV when administered to in vitro stimulated human T cells and in vivo mouse model for a type 1 cytokine-mediated immune response.
Early prediction of response to 40 KDA peginterferon alfa-2A (Pegasys) plus ribavirin (RBV) in patients with chronic hepatitis C (CHC). Abstract 716
BKG/objectives: The administration of 40 kDa peginterferon alfa-2a is associated with early viral response (EVR) in the majority of treated individuals. The failure to achieve an early virologic response (at least HCV RNA decrease by 2 log10 from baseline) by week 12 is highly predictive of lack of a sustained virologic response in patients treated with 40 kDa peginterferon alfa-2a monotherapy or the combination of 40 kDa peginterferon alfa-2a with ribavirin. The objective of this study was to determine the positive and negative predictive values (PPV and NPV) for SVR using the EVR in patients treated with combination 40 kDa peginterferon alfa-2a and ribavirin.
Methods: Retrospective analysis on data obtained from large (n = 453), prospective study comparing three different treatment regimens:
(a) 40 kDa peginterferon alfa-2a and ribavirin
(b) 40 kDa peginterferon alfa-2a and placebo
(c) IFN a-2b and RBV.
EVR-HCV RNA below level of detection (< 600 IU/ML) or > 2 log10 decrease in HCV RNA between baseline and week 12.
Adherence to therapy- evaluated to determine if subjects received at least 80% of assigned treatment.
Results: Overall 86% of treated patients had an EVR and 56% achieved an SVR. The EVR-PPV was 65% and the EVR-NPV was 97%. Subjects with an EVR who were adherent to 80% of the prescribed medication SVR was 75% compared to only 48% in EVR patients who did not maintain that level of adherence. EVR achieved in 81% of genotype 1 patients and in 97% of genotype 2 or 3 patients.
|
|
| |
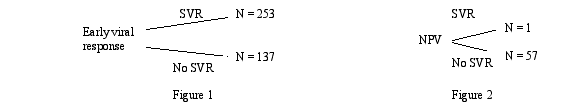 |
|
| |
Figure 1 demonstrates the likelihood of a sustained viralogic response among those individuals who achieved a EVR. Figure 2 shows the negative predictive value (NPV), the likelihood of not achieving an SVR among those individuals whom did not have an EVR.
(EDITORIAL NOTE: 67% of genotype 1 who were >80% adherent had an SVR vs 40% who were <80% adherent. This compares to 46% SVR for genotype 1 (Pegasys/RBV). For genotype 2 there was no difference in response by adherence (86%) or nonadherence (88%). But the SVR in genotype 1 was 76% in the Pegasys/RBV study).
Conclusions: EVR is highly predictive of achievement of SVR (PPV = 65%) and failure to achieve a EVR is highly predictive of therapeutic failure (NPV = 97%).
Chronic hepatitis C in HIV/HCV coinfected patients: Liver histology, fibrosis, fibro and immunocompetent cells and in situ detection of HCV. Abstract #1061
BKG/objectives: The objective of this study was to evaluate possible reasons why coinfected patients may have worse prognosis in terms of liver disease compared to HCV monoinfected individuals.
Methods: 37 HIV/HCV coinfected individuals without significant immunodeficiency (CD4 >250) who are naive to anti-HCV therapy were evaluated using a variety of different tissue-based techniques including immunohistochemistry, in situ hybridization, and RT-PCR.
Results: Coinfected patients had a significant increase in intrahepatic CD3+ cells and a significant decrease in CD4+ cells compared to that in HCV monoinfected individuals.
Conclusions/commentary: Hepatic fibrosis in HIV/HCV coinfected individuals tended to be more severe than HCV monoinfected individuals.
Progression of hepatitis C virus (HCV) infection: Relation to intrahepatic and peripheral HCV specific CD4+ T cell responses and cytokine patterns. Abstract #1094
(Summary taken from abstract book).
BKG/objectives: Individuals coinfected with HCV and Schistosoma mansoni, a parasitic disease that results principally in a Th2 profile, have an accelerated course of disease. This study evaluated whether the immune profile early in the course of HCV may explain different disease progression rates.
Methods: Twenty-two individuals with persistent HCV viremia after acute infection had analysis of HCV-specific CD4 and CD8 immune responses as determined by CD4 proliferative assay and IFN-g, TNF-a and IL-10 enzyme-linked immunospot assay (ELISPOT). Two liver biopsies were performed on all study participants, at baseline and at the end of follow-up.
Results: The fibrosis progression rate was significantly different in coinfected vs. monoinfected individuals (0.58/yr vs. 0.08/yr, p < 0.001) respectively. At baseline, 8/10 HCV monoinfected and 5/12 coinfected individuals produced significant numbers of HCV-specific, TNF-a and IFN-g producing CD4+ T cells. At baseline, these cells were comparable in vigor, frequency, and breadth between the intrahepatic and peripheral compartments. At the end of follow-up, HCV-specific CD4 responses were focused in the liver and differed between the two compartments with respect to frequency, strength, breadth, and cytokine production. Seven of ten monoinfected (produced primarily TNF-a and IFN-g) and 4/12 coinfected (produced only IL-10) compared to 4/10 monoinfected individuals who produced primarily TNF-a and IFN-g and 1/12 coinfected individuals (who produced IL10) in peripheral blood. HCV-specific CD4+ T cell responses at baseline inversely correlated with the fibrosis progression rate.
Conclusions/commentary: The authors concluded that HCV specific immune responses compartmentalize to the liver once the infection becomes persistent, and failure to develop a strong Th1 response early in the infection enhances the likelihood of progression to chronicity.
Impact of HIV coinfection on the age and the cause of death in patients with HCV infection. Abstract #1095
BKG/objectives: The goal of this study was to determine the impact of HIV infection on the relationship between age and cause of death in HCV-infected individuals with cirrhosis.
Methods: 445 HCV infected individuals were followed between 1998 and 2001 for a mean of 34 months (range 1-206). Fifty-four of the subjects were coinfected with HIV and HCV, 95 had decompensated HCV cirrhosis, and 261 of 445 received at least 3 months of anti-HCV therapy during the follow-up period.
Results: Complications occurred in 191/445 as demonstrated in the table below:
|
|
| |
 |
|
| |
Before 60 years of age, death was related to liver failure in 60%, to variceal bleeding in 24% and to hepatocellular carcinoma in 16%. After 60 years of age, death was related to liver failure in 27%, to variceal bleeding in 3% and hepatocellular carcinoma in 64%. HIV infected individuals died at an earlier age than HCV monoinfected individuals with 80% dying before age 60 and 65% dying before age 50 years. Among HIV infected individuals, there was a 35% likelihood of dying in the first year, 52% in the third year, and 67% in the fifth year after the first cirrhosis-related complication.
Conclusions/commentary: Age has a negative affect on HCV course in HIV/HCV coinfected individuals with HIV/HCV coinfected individuals dying earlier and more frequently than HCV monoinfected individuals, indicating that anti-HCV treatment should be initiated as early as possible in coinfected patients.
Hepatitis C virus (HCV)-specific immune responses and viral load in HIV-1/HCV coinfected individuals before and after interferon-a 2A therapy. Abstract #1252
BKG/objectives: The goal of this study was to evaluate the association between HCV specific immune response and successful outcome to anti-HCV therapy in HIV/HCV coinfected individuals.
Methods: Enzyme-linked immunospot assays (ELISPOT) were performed on seven HIV/HCV coinfected to two different portions of the HCV genome (core-NS3 and NS3-NS5) on individuals initiating anti-HCV therapy with interferon alfa-2a. HCV RNA was determined and PBMCs were isolated at baseline and one month after initiation of IFN.
Results: After one month of therapy HCV RNA decreased below assay detection (1000 copies/ml) and immune responses that were 3X and 43X background. Three subjects who had partial responses to IFN (at least one log decline during the first month) had no response against HCV at baseline. One month after initiating IFN, these subjects developed responses that were 9X, 3X, and 3X background in the C-NS3 region. Three additional patients developed neither virologic nor immunologic responses.
Conclusions/commentary: These data suggest that the strength and focus of the HCV specific immune responses may be predictive of a virologic response in HIV/HCV coinfected individuals being treated with IFN alfa.
|
|
| |
|
 |
 |
|
|