 |
 |
 |
| |
PrEP at IAS Vancouver 2015 July 19-22.....Daily PrEP Appears Most Effective....more NATAP PrEP IAS Reports coming
|
| |
| |
IAS: HIV Control Through Treatment Durably Prevents Heterosexual Transmission of Virus - NIH HPTN 052 Study - (07/21/15)
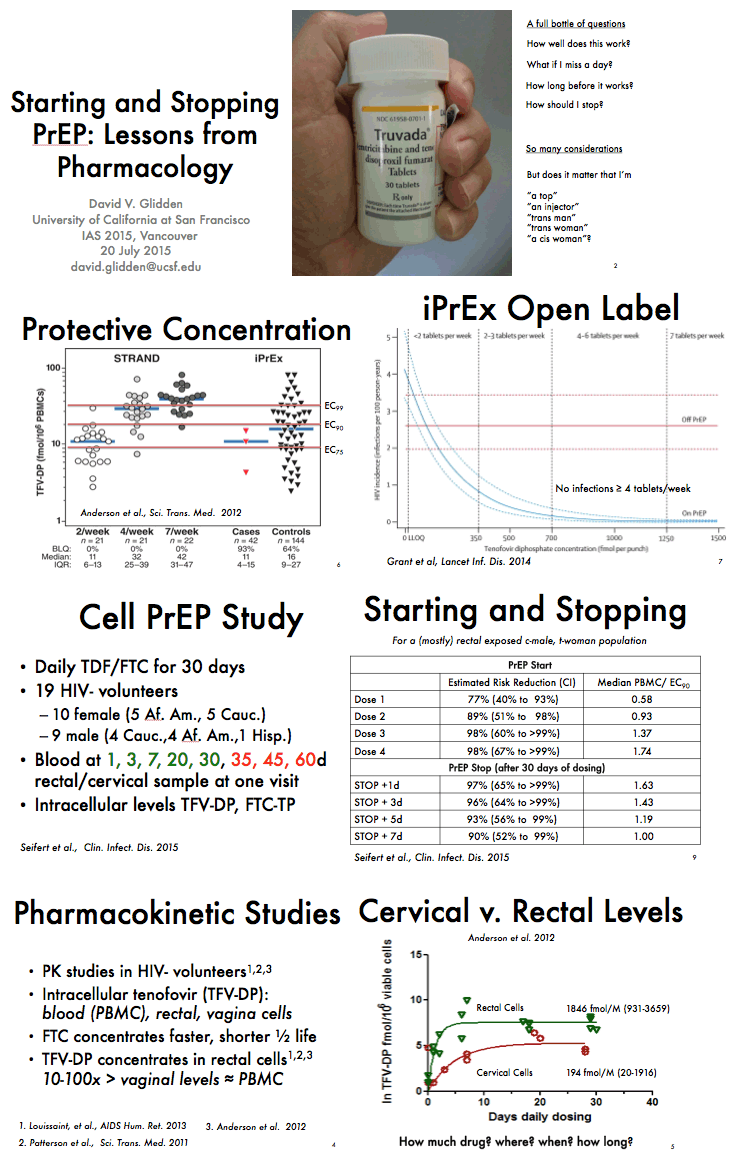
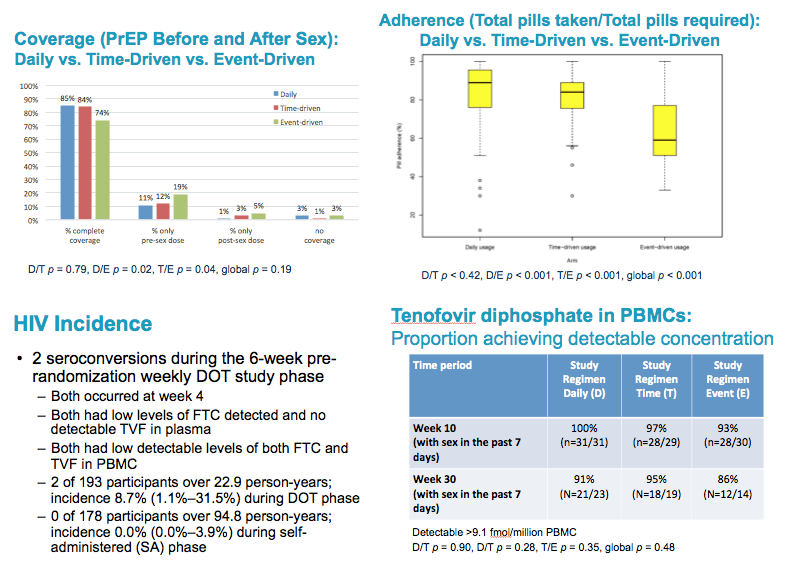
from Jules: there were a number of PrEP studies at IAS Vancouver July 19-22 2015 experimenting with non-daily & event driven dosing, I have reviewed most of these presentations & it appears daily dosing is the most effective prevention method & adherence is important as well, below the pics from the slide presentations at IAS is a published article that was referred to be a speaker at IAS that addresses why daily dosing provides the best coverage & that 4-6 doses a week appear to come close. Three oral presentations at IAS presented study results from 3 studies conducted by HPTN (HIV Prevention Trials Network)- one study in Harlem NY among MSM, immediately below (select slides); the 2nd study in women in South Africa; and the 3rd study in Bangkok among MSM. All 3 studies, here is study design, after a DOT, direct observed therapy, lead-in - there were 3 approaches to dosing: daily, Time-Driven & Event driven: ADDITIONAL PrEP Reports coming from NATAP.
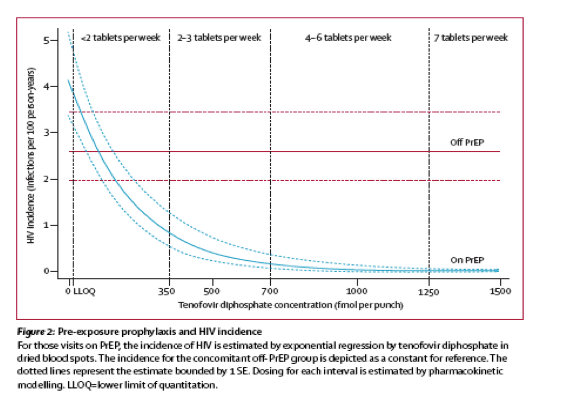
"In summary, this study indicates that approximately 1 week of daily PrEP is expected to confer high PrEP activity for MSM. Although a high level of protection may persist for several days after stopping PrEP from steady state, 4 weeks of continued PrEP dosing is reasonable relative to the last potential HIV exposure."......."Other studies also support approximately 1 week of lead-in time for starting PrEP in MSM.......Taken together, a high level of PrEP activity was achieved by 5-7 doses, indicating that, for optimal benefit, PrEP should be started approximately 1 week before it is needed. It is important to note that this analysis was based on data in MSM (iPrEx), so these results should not be extrapolated to women, heterosexual men, or other routes of transmission. Understanding and applying PK/PD analyses for PrEP in women, and populations other than MSM, is an urgent research priority......Conclusions.High PrEP activity for MSM was achieved by approximately 1 week of daily dosing. Although effective intracellular drug concentrations persist for several days after stopping PrEP, a reasonable recommendation is to continue PrEP dosing for 4 weeks after the last potential HIV exposure, similar to recommendations for postexposure prophylaxis."
Following the IAS slides below you will find:
2 Published Studies on Best Dosing Practices
--------------------
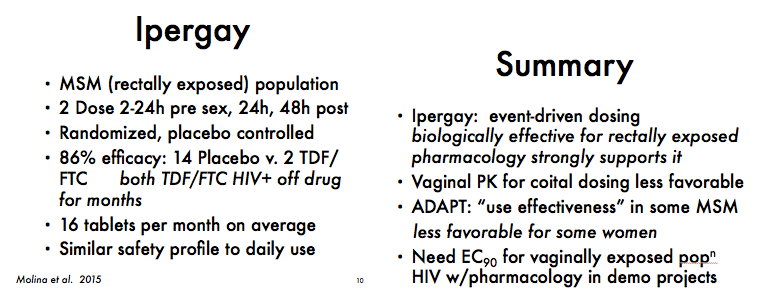
IAS: Ipergay Analysis Suggests Both Pre- and Postsex TDF/FTC PrEP Doses Needed for Full Protection Ð
----------------------
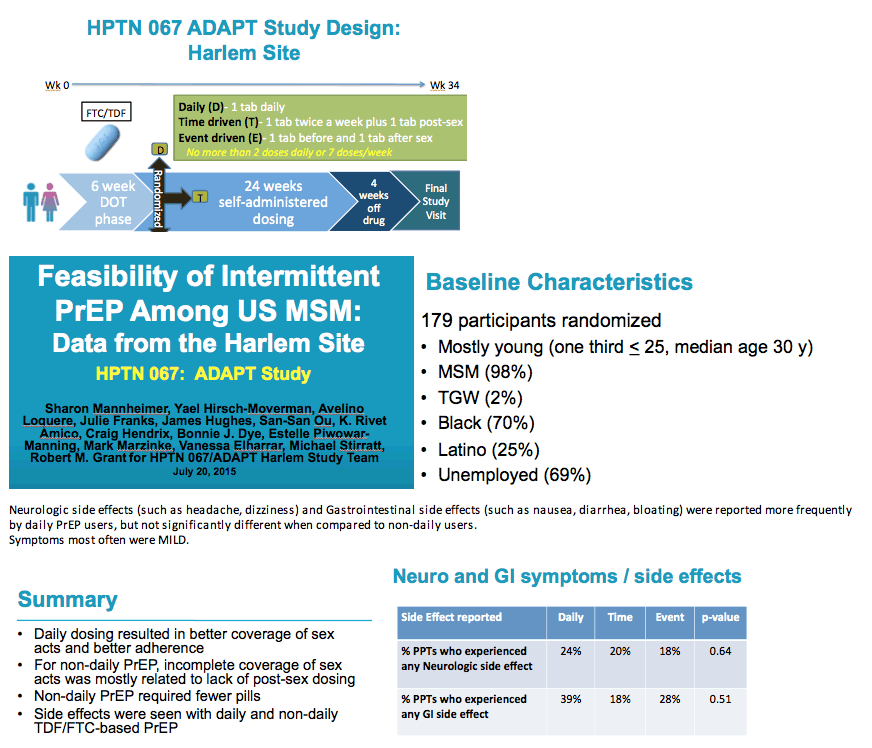

Neurologic side effects (such as headache, dizziness) and Gastrointestinal side effects (such as nausea, diarrhea, bloating) were reported more frequently by daily PrEP users, but not significantly different when compared to non-daily users.
Symptoms most often were MILD.
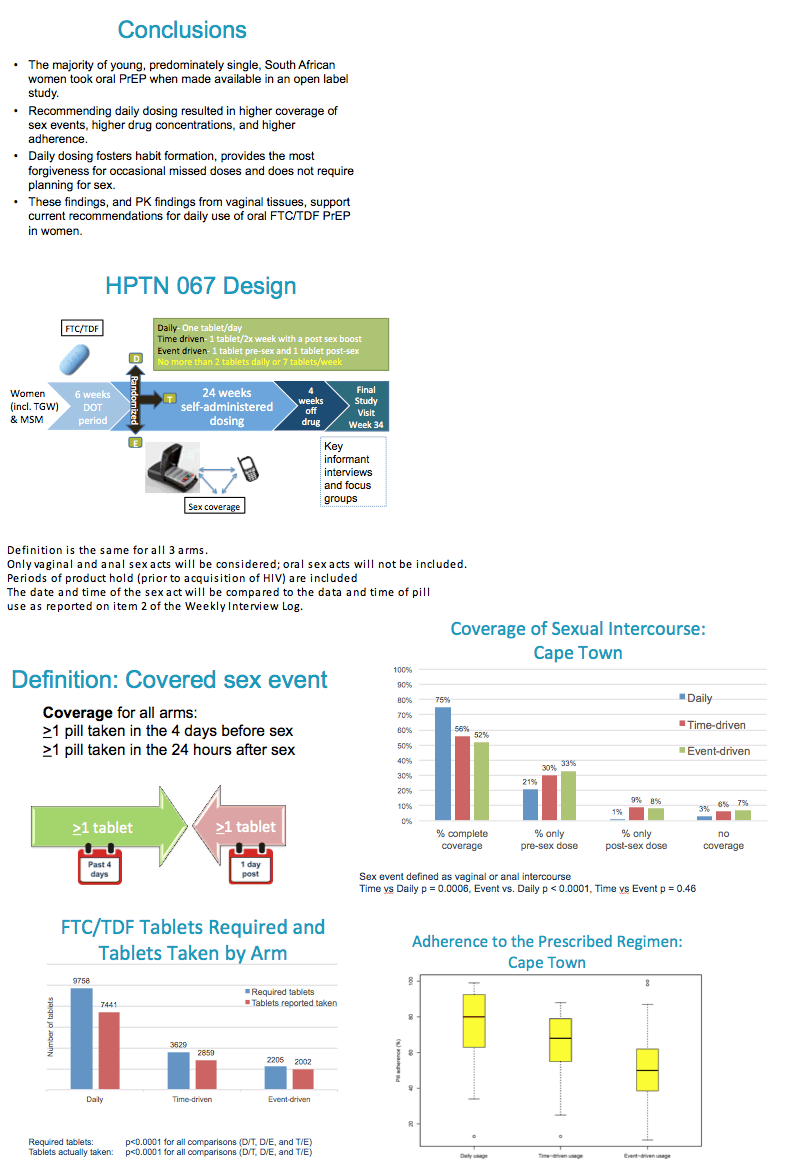
Coverage of sex acts was one of the primary study endpoints:.
"Complete coverage" was defined as taking at least 1 PrEP tablet within 4 days before sex and at least 1 PrEP tablet with 24 hours after sex.
Daily arm participants reported complete coverage of 66% of sex acts, which was significantly higher than in the Time-driven and Event-Driven arms which had complete coverage for 47% & 52% of sex acts, respectively.
In cases of partial coverage of sex acts, participants in all 3 arms were more likely to complete the pre-sex dose / more likely to miss the post-sex dose.
Adherence to the assigned study arm dosing regimen was significantly higher in the daily arm, compared to the time-driven and event-driven arms
65% vs 46% vs 41%

---------------------------------------------
2 Published Studies on Best Dosing Practices
Adherence/how many Tenofovir PrEP pills worked in study - Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study.....iPrEx open-label extension.......http://www.natap.org/2014/HIV/092914_05.htm.......The Lancet Infectious Diseases, Bob Grant, September 2014
Dose Response for Starting and Stopping HIV Preexposure Prophylaxis for Men Who Have Sex With Men - (02/23/15)
PrEP Dosing Required to Achieve and Maintain Intracellular Drug Concentrations
"In summary, this study indicates that approximately 1 week of daily PrEP is expected to confer high PrEP activity for MSM. Although a high level of protection may persist for several days after stopping PrEP from steady state, 4 weeks of continued PrEP dosing is reasonable relative to the last potential HIV exposure."......."Other studies also support approximately 1 week of lead-in time for starting PrEP in MSM."
Clin Infect Dis. Sharon M. Seifert, (2015)
"This study evaluated pharmacokinetic data from daily dosing in Cell-PrEP with a pharmacodynamic model for HIV risk reduction from iPrEx, and calculated an inferred HIV risk reduction that reached 99% by 5 days of daily dosing. This 99% value provides an average estimate based on the set of TFV-DP concentrations from Cell-PrEP participants for each daily dose, suggesting that most concentrations conferred nearly 100% efficacy from the fifth dose onward. Sensitivity analyses, including one in which data from female participants were excluded, showed consistent results.
A second, more conservative approach, showed that the majority of Cell-PrEP participants exceeded the iPrEx EC90 by the fifth or seventh doses, approximately 80% and 90%, respectively, and that the maximum proportion was achieved by the 13th dose (98%). Reaching the highest proportion by the 13th dose was consistent with the pharmacokinetic profile of TFV-DP in PBMCs, which demonstrated that >90% steady state was achieved after approximately 12 doses (Figure 3). FTC-TP in PBMCs achieved steady state more rapidly-92% after 5 doses and 97% after 7 doses.
A third approach focused on TFV-DP (ln transformed) at an important site of action in MSM, in rectal mononuclear cells, showing that 88% and 94% of steady state were achieved after 5 and 7 doses, respectively (Figure 2). FTC-TP concentrations in rectal mononuclear cells also appeared to reach steady state by this time.
Taken together, a high level of PrEP activity was achieved by 5-7 doses, indicating that, for optimal benefit, PrEP should be started approximately 1 week before it is needed. It is important to note that this analysis was based on data in MSM (iPrEx), so these results should not be extrapolated to women, heterosexual men, or other routes of transmission. Understanding and applying PK/PD analyses for PrEP in women, and populations other than MSM, is an urgent research priority.
The considerations for how to safely stop PrEP after the last potential HIV exposure are not as straightforward. The present study found that high PrEP activity was evident for several days after dosing was stopped. The inferred risk reduction exceeded 90% for 7 days, and 80% of participants remained above the iPrEx EC90 for 2 days after stopping PrEP. However, an important consideration for discontinuing PrEP is how long it takes for HIV to be completely cleared from the body following the last potential exposure. There appears to be little consensus on this issue given that several variables may affect the HIV clearance process, such as whether HIV is endocytosed into Langerhans cells (where it may persist for days), and/or adhered to follicular dendritic cells, and/or whether early cycles of HIV replication occur [16, 17]. As early stages of viral replication occur, longer times are required to completely clear the virus, and the chance for establishing latent infection increases. This was demonstrated in early postexposure prophylaxis (PEP) studies in animals that showed 50% vs 100% efficacy for 10 days vs 28 days of tenofovir dosing when started 24 hours after intravenous simian immunodeficiency virus exposure [16]. These considerations underlie the current PEP recommendations to treat potential HIV exposures for 28 days [18].
PrEP differs from PEP in that early replication is presumably blocked by PrEP, as long as PrEP activity is high, perhaps allowing for a faster HIV clearance rate in the setting of PrEP. Similar efficacy was found in animal studies that compared continued PrEP dosing for 28 days after the last viral inoculation vs discontinued dosing after the last viral inoculation [19]. These considerations suggest that shorter durations of dosing might be adequate for PrEP following the last potential HIV exposure, but not enough information is available to make specific recommendations. This should be an area of future research. Until then, a conservative recommendation would be to continue dosing for 4 weeks after the last potential HIV exposure."
"Results.Twenty-one HIV-uninfected adults participated in Cell-PrEP. The inferred HIV risk reduction, based on PBMC TFV-DP concentration, reached 99% (95% confidence interval [CI], 69%-100%) after 5 daily doses, and remained >90% for 7 days after stopping drug from steady-state conditions. The proportion of participants reaching the 90% effective concentration (EC90) was 77% after 5 doses and 89% after 7 doses. The percentage of steady state for natural log [TFV-DP] in rectal mononuclear cells was 88% (95% CI, 66%-94%) after 5 doses and 94% (95% CI, 78%-98%) after 7 doses.
Conclusions. High PrEP activity for MSM was achieved by approximately 1 week of daily dosing. Although effective intracellular drug concentrations persist for several days after stopping PrEP, a reasonable recommendation is to continue PrEP dosing for 4 weeks after the last potential HIV exposure, similar to recommendations for postexposure prophylaxis."
|
| |
|
 |
 |
|
|