 |
 |
 |
| |
|
Cabotegravir (CAB) Long-Acting (LA)
Phase 3 (Ph3) PrEP Dose Selection Based on Population Pharmacokinetics (PPK) in Healthy and HIV-Infected Adults
|
| |
| |
HIVR4P: Acceptability of long-acting injectable cabotegravir (CAB LA) in HIV-uninfected individuals: HPTN 077 - (10/25/18)
HIVR4P: Tail-phase safety, tolerability and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected individuals: HPTN 077 final results - - (10/25/18)
During Q&A which you can g=here on webcast question was asked about using oral CAB during injection interruptions and response was yes this can e accommodated and will be in FDA submission & indications.
Reported by Jules Levin
HIVR4P - HIV Research for Prevention (HIVR4P), October 21-25, 2018, Madrid
Kelong Han,1 Parul Patel,2 Mark Baker,3 David Margolis,2 William Spreen,2 Alex Rinehart,2 Raphael Landovitz,4 Susan Ford5
WEBCAST:
http://webcasts.hivr4p.org/console/player/40423?mediaType=slideVideo&&crd_fl=1&ssmsrq=1540731797528&ctms=5000&csmsrq=905
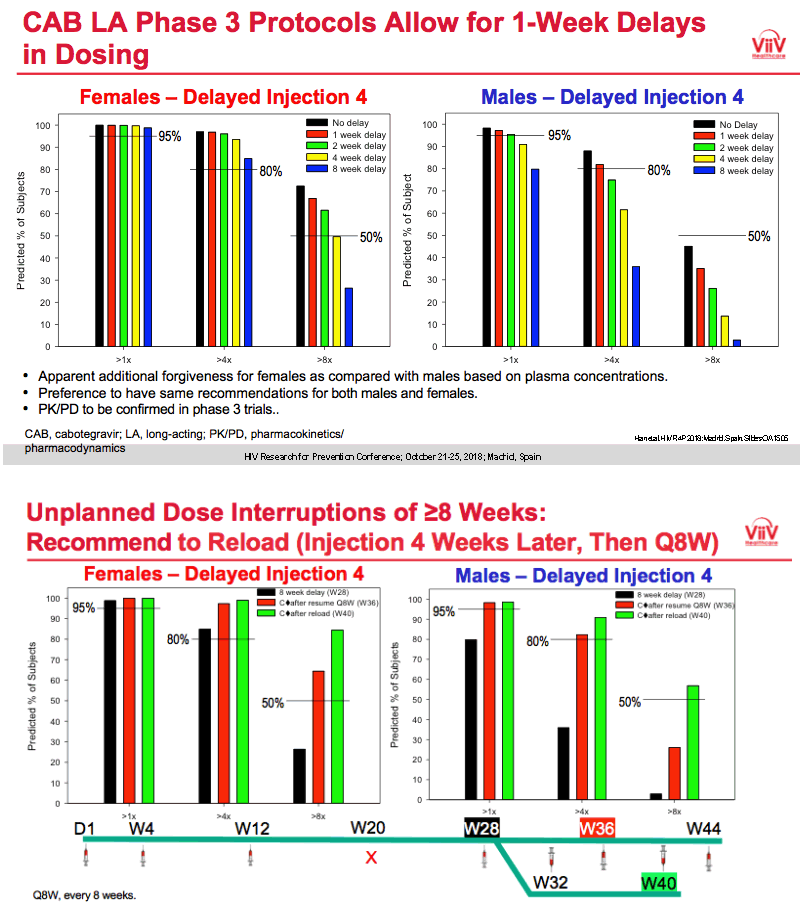
|
| |
|
 |
 |
|
|