 |
 |
 |
| |
Sharp Drops in HIV Load After 10 Days of Capsid Inhibitor Monotherapy
|
| |
| |
10th IAS Conference on HIV Science (IAS 2019), July 21-24, 2019, Mexico City
Mark Mascolini
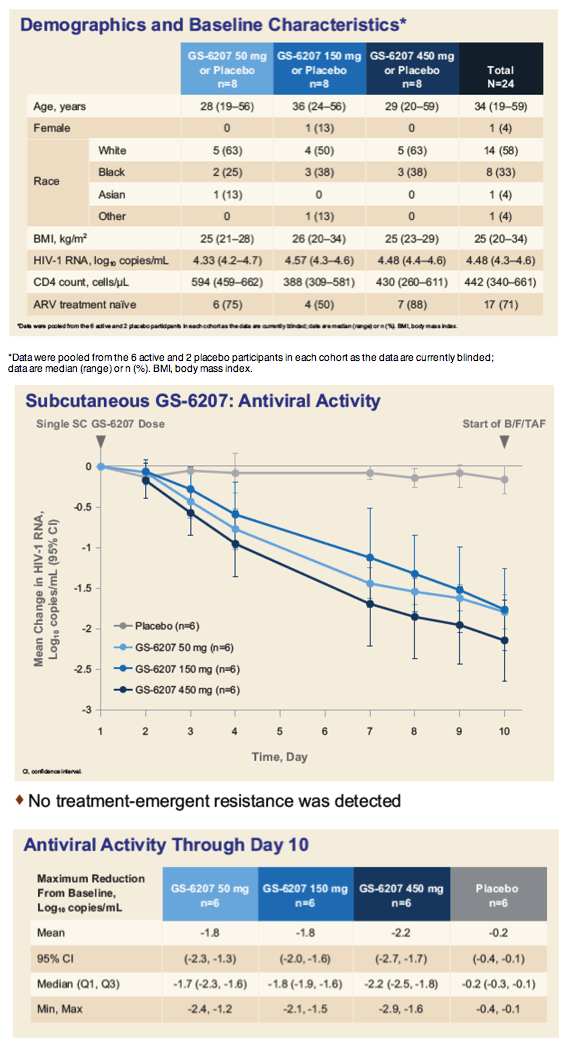
Viral loads in people with HIV dropped an average 1.8 to 2.2 log10 copies/mL (about 63- to 158-fold) with GS-6207, a novel HIV capsid inhibitor [1]. In this 24-person placebo-controlled dose-ranging trial, there were no grade 3 or 4 adverse events with any dose of GS-6207 and no adverse events leading to stopping treatment.
GS-6207 has a novel mechanism of action, interrupting activity of the HIV capsid, the protein that surrounds the genetic material and essential enzymes of HIV. Previous research in HIV-negative people found that a single subcutaneous dose of GS-6207 up to 450 mg maintains levels of the drug for more than 24 weeks [2]. The new phase 1b, double-blind, randomized, placebo-controlled trial assessed GS-6207 for the first time in people with HIV.
Participants needed a viral load between 5000 and 400,000 copies, a CD4 count above 200, and no experience with HIV capsid inhibitors or integrase inhibitors. Researchers randomized participants to 50, 150, or 450 mg of GS-6207 or to placebo for 10 days (6 participants in each study arm). At that point everyone began a regimen of single-tablet bictegravir, emtricitabine, and tenofovir alafenamide (TAF). The primary endpoint was drop in viral load through day 10.
Median age of the 24 participants stood at 34 years, 14 (58%) were white, 8 (33%) black, and 2 (8%) Asian or other. The study group included 1 woman. Seventeen of 24 participants (71%) were naive to antiretrovirals. Initial viral loads averaged 4.48 log10 copies/mL (about 30,000 copies) and did not vary much from arm to arm.
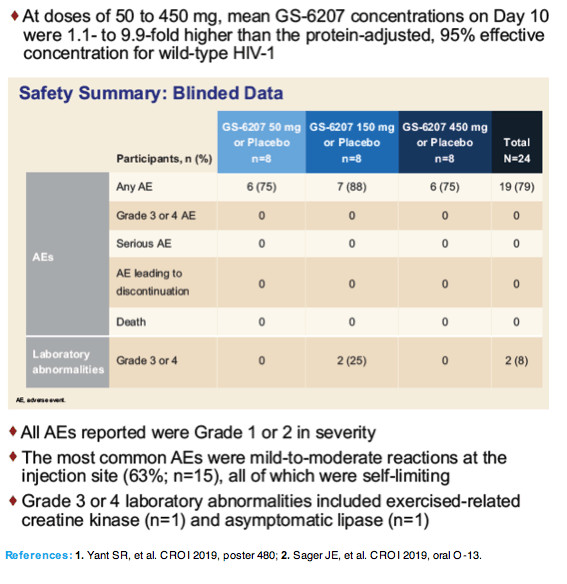
At day 10 viral load dropped an average 1.8 log in the 50-mg group, 1.8 log in the 150-mg group, and 2.2 log in the 450-mg group, compared with 0.2 log in the placebo group. All differences between GS-6207 groups and placebo were highly statistically significant (P < 0.0001). In the 50- and 450-mg groups, average GS-6207 concentrations on day 10 stood 1.1- to 9.9-fold above the protein-adjusted 95% effective concentration against wild-type (nonmutant) HIV.
All adverse events were grade 1 or 2. Self-limiting mild to moderate injection site reactions affected 15 people (63%). There were no grade 3 or 4 adverse events and 2 grade 3 or 4 lab abnormalities (exercise-related creatine kinase and asymptomatic lipase abnormalities). There were no serious adverse events or events leading to discontinuation arose.
The researchers noted that their preliminary findings represent the first proof of concept for anti-HIV activity of capsid inhibition in humans.
References
1. Daar ES, McDonald C, Crofoot G, et al. Safety and antiviral activity over 10 days following a single dose of subcutaneous GS-6207, a first-in-class, long-acting HIV capsid inhibitor in people living with HIV. 10th IAS Conference on HIV Science (IAS 2019), July 21-24, 2019, Mexico City. Abstract LBPEB13.
2. Sager JE, Begley R, Rhee M, et al. Safety and PK of subcutaneous GS-6207, a novel HIV-1 capsid inhibitor. Conference on Retroviruses and Opportunistic Infections. March 4-7, 2019, Seattle. Abstract 141.
http://www.croiconference.org/sessions/safety-and-pk-subcutaneous-gs-6207-novel-hiv-1-capsid-inhibitor
CROI: SAFETY AND PK OF SUBCUTANEOUS GS-6207, A NOVEL HIV-1 CAPSID INHIBITOR (03/08/19)
CROI: GS-6207, A POTENT AND SELECTIVE FIRST-IN-CLASS LONG-ACTING HIV-1 CAPSID INHIBITOR


|
| |
|
 |
 |
|
|