| |
HIV infection is associated with an increased risk of PAD, Peripheral Artery Disease
|
| |
| |
Download the PDF
This risk increases in a dose-response fashion with increasing HIV viral loads or lowering of CD4 cell counts. In fact, HIV+ veterans with sustained CD4 counts <200 cells/mm3 have nearly twice the risk of PAD whereas HIV+ veterans with sustained CD4 counts ≥500 cells/mm3 do not have an excess risk of incident PAD events compared with uninfected veterans. Mortality rates after PAD diagnosis are high regardless of HIV status; however, for those with low CD4 cell counts or unsuppressed HIV viremia, ≈50% are deceased within 5 years. In contrast, rates of amputation after incident PAD events diagnosis are similar for both HIV+ and uninfected veterans.. patients with PAD experience a significant increase in adverse limb events (eg, claudication, critical limb ischemia, acute limb ischemia, and amputation) as well as mortality
Peripheral arterial disease (PAD) in the legs or lower extremities is the narrowing or blockage of the vessels that carry blood from the heart to the legs. It is primarily caused by the buildup of fatty plaque in the arteries, which is called atherosclerosis. PAD can happen in any blood vessel, but it is more common in the legs than the arms.
The classic symptom of PAD is pain in the legs with physical activity, such as walking, that gets better after rest. However, up to 4 in 10 people with PAD have no leg pain.1 Symptoms of pain, aches, or cramps with walking (claudication) can happen in the buttock, hip, thigh, or calf.2
If you have symptoms of PAD, your doctor may do an ankle brachial index (ABI), which is a noninvasive test that measures the blood pressure in the ankles and compares it with the blood pressure in the arms at rest and after exercise. Your doctor may also do imaging tests such as ultrasound, magnetic resonance angiography (MRA), and computed tomographic (CT) angiography.1-3
Both men and woman are affected by PAD; however, African Americans have an increased risk of PAD. Hispanics may have similar to slightly higher rates of PAD compared with non-Hispanic white people. Approximately 6.5 million people age 40 and older in the United States have PAD.1
Other health conditions and disorders of arteries can mimic the symptoms of PAD, and not all PAD is due to atherosclerosis.2,3
If you have PAD, you are at risk for developing coronary artery disease and cerebrovascular disease, which could lead to a heart attack or stroke.4
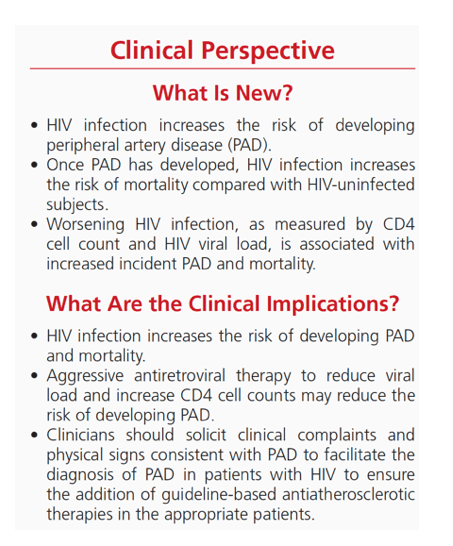
"When limiting the population to HIV+ veterans, older age, hypertension, diabetes mellitus, elevated triglyceride concentrations, current smoking, hepatitis C virus coinfection, chronic kidney disease, anemia, and chronic obstructive pulmonary disease increased the hazard for incident PAD events (Table 3). Similarly, time-updated low CD4 cell counts and unsuppressed HIV viral load were both associated with an increased risk of incident PAD events (Table 3).....In our study, atherogenic risk factors, including older age, hypertension, diabetes mellitus, current smoking, and advanced chronic kidney disease increased the risk of PAD.
However, the HIV+ patients with PAD did not have a risk factor profile more adverse than those PAD patients without HIV. These findings suggest that HIV, per se, impairs vascular function and promotes systemic atherosclerosis. HIV-infected patients have been shown to manifest endothelial dysfunction,23 platelet activation,24 increased inflammation,25 monocyte activation, and a prothrombotic tendency.25 Other possibilities include the use of earlier generation antiretroviral therapy with the development of adverse metabolic changes. The exact mechanisms by which HIV increases atherogenesis in general and PAD specifically remain unclear.”
In the general population, PAD affects 8 to 10 million people in the United States and represents the second most common clinical manifestation of atherosclerosis after MI.26 In contrast to MI or stroke, patients with PAD experience a significant increase in adverse limb events (eg, claudication, critical limb ischemia, acute limb ischemia, and amputation) as well as mortality.27 In this study, mortality rates among those with incident PAD events were high regardless of HIV status. For those with poorly controlled HIV infection the mortality rates were even higher. In contrast, amputation rates after PAD did not differ by HIV status. Whether major adverse limb events are truly the same for HIV+ and uninfected veterans is not clear because we examined the least common phenotype within the major adverse limb events category. The fact that microvascular disease (ie, nephropathy and neuropathy) in PAD is associated with an increased risk of major adverse limb events and that HIV+ people are at risk for microvascular disease supports further examination of major adverse limb event outcomes among HIV+ people with PAD.
HIV infection is associated with an increased risk of PAD. This risk persists despite a similar or reduced atherosclerotic risk factor burden among HIV+ veterans. This risk increases in a dose-response fashion with increasing HIV viral loads or lowering of CD4 cell counts. In fact, HIV+ veterans with sustained CD4 counts <200 cells/mm3 have nearly twice the risk of PAD whereas HIV+ veterans with sustained CD4 counts ≥500 cells/mm3do not have an excess risk of incident PAD events compared with uninfected veterans. Mortality rates after PAD diagnosis are high regardless of HIV status; however, for those with low CD4 cell counts or unsuppressed HIV viremia, ≈50% are deceased within 5 years. In contrast, rates of amputation after incident PAD events diagnosis are similar for both HIV+ and uninfected veterans.
The limited data involving HIV and PAD20 likely reflects the underdiagnosis and treatment of PAD from the general population to patients infected with HIV.21 The dearth of diagnostic scrutiny squanders 2 therapeutic opportunities: first, the occasion to institute broad atherosclerotic risk factor modification therapies, and second, the chance to ensure the use of antiretroviral therapy in a patient population likely to require frequent medical care.22
Our data suggest that HIV infection is a risk factor for incident PAD events in addition to and independent of the well-established risk factors for atherosclerosis. The risk of PAD is highest among those with high HIV viral loads and low CD4 cell counts. In contrast, HIV-infected people with sustained high CD4 cell counts do not have an excess risk of PAD compared with uninfected people. After the development of PAD, mortality rates are high regardless of HIV status but highest for those with untreated HIV infection. These results suggest that patients with HIV and PAD require aggressive treatment of both conditions to decrease mortality. Moreover, novel treatments may be required to reduce the increased mortality of HIV patients who are seemingly well-controlled.
Heightened Risk of Peripheral Arterial Disease With HIV in Veterans
IAC 2017
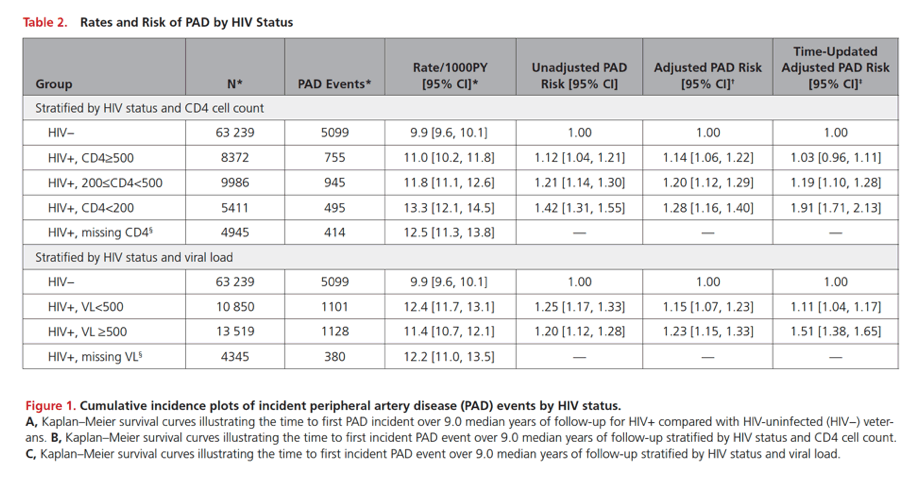
In adjusted analysis HIV infection was independently associated with incident peripheral arterial disease regardless of CD4 bracket (hazard ratios [HR] ranging from about 1.2 to almost 1.4) or viral load (HR ranging from almost 1.2 to 1.3).
In the adjusted analysis with time-updated CD4 count, HIV-positive veterans with 200-499 CD4s versus 500 or more had a 13% higher risk of peripheral arterial disease (HR 1.13, 95% confidence interval [CI] 1.02 to 1.25) while veterans with fewer than 200 CD4s versus 500 had a 50% higher risk (HR 1.5, 95% CI 1.30 to 1.74). An adjusted analysis with time-updated viral load determined that veterans with a load of 500 copies or more versus a lower load had almost a 20% higher risk of peripheral arterial disease (HR 1.19, 95% CI 1.05 to 1.34).
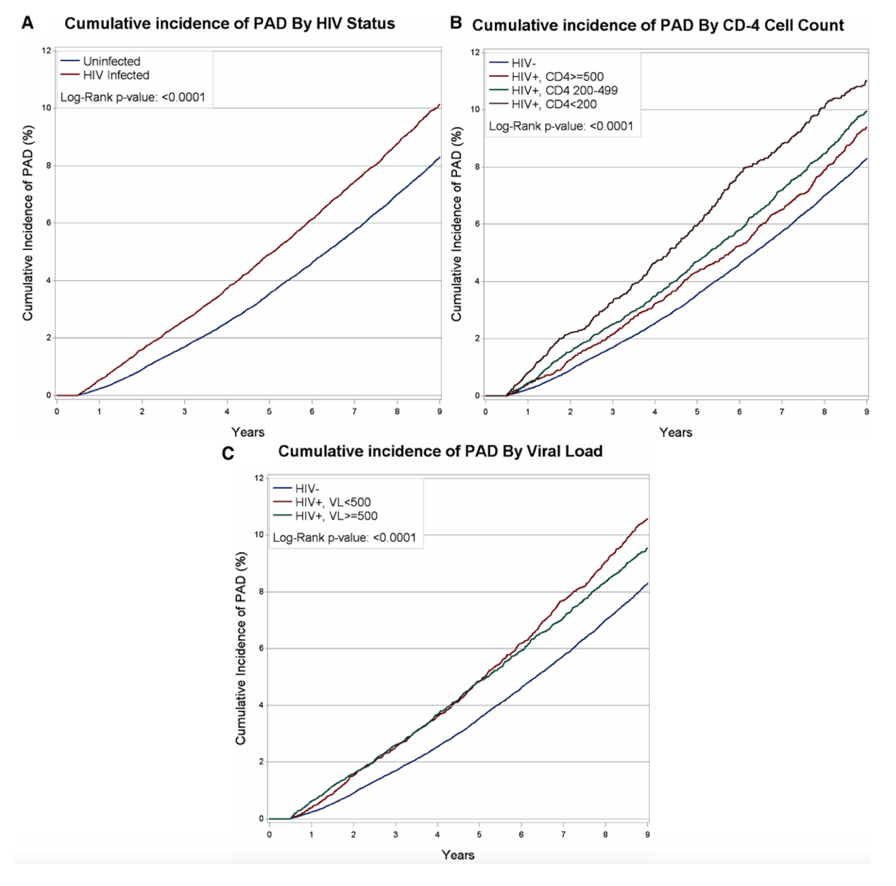
Analysis of survival through 8 years after peripheral arterial disease diagnosis excluded veterans with missing CD4 or viral load data. Compared with HIV-negative veterans, survival was shorter in veterans with more than 500 CD4 cells at diagnosis, shorter still in those with 200 to 500 CD4s, and shortest in those with fewer than 200 CD4s (P < 0.0001).Similarly, through 8 years of follow-up after diagnosis, survival was shorter with than without HIV in those with a viral load below 500 copies at diagnosis and shorter still in those with a higher viral load (P < 0.0001).
The VACS team concluded that, in veterans with HIV, "those with higher HIV viral loads and lower CD4 cell counts have the highest risk of peripheral arterial disease." And after diagnosis, mortality is greatest in HIV-positive veterans with immunodeficiency or a detectable viral load.
---------------------------
published
Circulation. 2018

Abstract
Background:
The effect of human immunodeficiency virus (HIV) on the development of peripheral artery disease (PAD) remains unclear. We investigated whether HIV infection is associated with an increased risk of PAD after adjustment for traditional atherosclerotic risk factors in a large cohort of HIV-infected (HIV+) and demographically similar HIV-uninfected veterans.
Methods:
We studied participants in the Veterans Aging Cohort Study from April 1, 2003 through December 31, 2014. We excluded participants with known prior PAD or prevalent cardiovascular disease (myocardial infarction, stroke, coronary heart disease, and congestive heart failure) and analyzed the effect of HIV status on the risk of incident PAD events after adjusting for demographics, PAD risk factors, substance use, CD4 cell count, HIV-1 ribonucleic acid, and antiretroviral therapy. The primary outcome is incident peripheral artery disease events. Secondary outcomes include mortality and amputation in subjects with incident PAD events by HIV infection status, viral load, and CD4 count.
Results:
Among 91 953 participants, over a median follow up of 9.0 years, there were 7708 incident PAD events. Rates of incident PAD events per 1000 person-years were higher among HIV+ (11.9; 95% confidence interval [CI], 11.5-12.4) than uninfected veterans (9.9; 95% CI, 9.6-10.1).
After adjustment for demographics, PAD risk factors, and other covariates,HIV+ veterans had an increased risk of incident PAD events compared with uninfected veterans (hazard ratio [HR], 1.19; 95% CI, 1.13-1.25). This risk was highest among those with time-updated HIV viral load >500 copies/mL (HR, 1.51; 95% CI, 1.38-1.65) and CD4 cell counts <200 cells/mm3 (HR, 1.91; 95% CI, 1.71-2.13). In contrast, HIV+ veterans with time updated CD4 cell count ≥500 cells/mm3 had no increased risk of PAD (HR, 1.03; 95% CI, 0.96-1.11). Mortality rates after incident PAD events are high regardless of HIV status. HIV infection did not affect rates of amputation after incident PAD events.
Conclusions:
Infection with HIV is associated with a 19% increased risk of PAD beyond that explained by traditional atherosclerotic risk factors. However, for those with sustained CD4 cell counts <200 cells/mm3, the risk of incident PAD events is nearly 2-fold higher whereas for those with sustained CD4 cell counts ≥500 cells/mm3 there is no excess risk of incident PAD events compared with uninfected people.
The advent of effective antiretroviral therapy (ART) has significantly increased the survival of patients infected with human immunodeficiency virus (HIV). Currently, the life expectancy of these patients approaches that of the general population.1,2 An increased risk in cardiovascular disease events is present even among well-treated patients with HIV.3 This fact may represent an important cause that prevents attainment of a normal life span.4The mechanisms underlying this excess risk of cardiovascular disease may include the inherent immune activation of HIV infection or other commonly acquired infections like hepatitis C, metabolic changes associated with some of the antiretroviral agents, and an increased prevalence of cardiovascular risk factors.5 The impact of HIV infection, independent of these other factors, is likely substantial. HIV infection increases rates of myocardial infarction (MI), even among those with few or no cardiovascular disease risk factors,3 as compared with uninfected people. Worse, standard medical therapy may not provide the same risk reduction as in the general population.6
Atherosclerosis is a systemic disease whose phenotype depends on the manifestation of clinical presentation.7 HIV infection is associated with an increased risk of MI and ischemic stroke.8 In contrast, there is sparse information concerning the relationship between HIV and the other major presentation of atherosclerosis: peripheral artery disease (PAD).9,10 Accordingly, we examined the association between HIV and PAD in an older, predominantly male population compared with a demographically and behaviorally similar uninfected population (eg, similar rates of substance use).

|
|
| |
| |
|
|
|